Defining and targeting patterns of T cell dysfunction in inborn errors of immunity
- PMID: 36189259
- PMCID: PMC9516113
- DOI: 10.3389/fimmu.2022.932715
Defining and targeting patterns of T cell dysfunction in inborn errors of immunity
Abstract
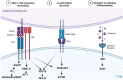
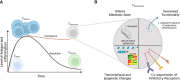
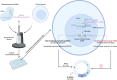
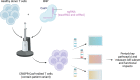
Inborn errors of immunity (IEIs) are a group of more than 450 monogenic disorders that impair immune development and function. A subset of IEIs blend increased susceptibility to infection, autoimmunity, and malignancy and are known collectively as primary immune regulatory disorders (PIRDs). While many aspects of immune function are altered in PIRDs, one key impact is on T-cell function. By their nature, PIRDs provide unique insights into human T-cell signaling; alterations in individual signaling molecules tune downstream signaling pathways and effector function. Quantifying T-cell dysfunction in PIRDs and the underlying causative mechanisms is critical to identifying existing therapies and potential novel therapeutic targets to treat our rare patients and gain deeper insight into the basic mechanisms of T-cell function. Though there are many types of T-cell dysfunction, here we will focus on T-cell exhaustion, a key pathophysiological state. Exhaustion has been described in both human and mouse models of disease, where the chronic presence of antigen and inflammation (e.g., chronic infection or malignancy) induces a state of altered immune profile, transcriptional and epigenetic states, as well as impaired T-cell function. Since a subset of PIRDs amplify T-cell receptor (TCR) signaling and/or inflammatory cytokine signaling cascades, it is possible that they could induce T-cell exhaustion by genetically mimicking chronic infection. Here, we review the fundamentals of T-cell exhaustion and its possible role in IEIs in which genetic mutations mimic prolonged or amplified T-cell receptor and/or cytokine signaling. Given the potential insight from the many forms of PIRDs in understanding T-cell function and the challenges in obtaining primary cells from these rare disorders, we also discuss advances in CRISPR-Cas9 genome-editing technologies and potential applications to edit healthy donor T cells that could facilitate further study of mechanisms of immune dysfunctions in PIRDs. Editing T cells to match PIRD patient genetic variants will allow investigations into the mechanisms underpinning states of dysregulated T-cell function, including T-cell exhaustion.
Keywords: CRISPR (clustered regularly interspaced short palindromic repeat)/Cas9 (CRISPR associated protein 9)-mediated genome editing; human immunology; inborn error of immunity (IEI); precision medicine; t cell exhaustion.
Copyright © 2022 Campos and Henrickson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All figures created with BioRender.com.
Figures
References
-
- Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. . Human inborn errors of immunity: 2019 update on the classification from the international union of immunological societies expert committee. J Clin Immunol (2020) 40:24–64. doi: 10.1007/s10875-019-00737-x - DOI - PMC - PubMed
-
- Chan AY, Leiding JW, Liu X, Logan BR, Burroughs LM, Allenspach EJ, et al. . Hematopoietic cell transplantation in patients with primary immune regulatory disorders (PIRD): A primary immune deficiency treatment consortium (PIDTC) survey. Front Immunol (2020) 11. doi: 10.3389/fimmu.2020.00239 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

