Reprogramming alveolar macrophage responses to TGF-β reveals CCR2+ monocyte activity that promotes bronchiolitis obliterans syndrome
- PMID: 36189800
- PMCID: PMC9525120
- DOI: 10.1172/JCI159229
Reprogramming alveolar macrophage responses to TGF-β reveals CCR2+ monocyte activity that promotes bronchiolitis obliterans syndrome
Abstract
Bronchiolitis obliterans syndrome (BOS) is a major impediment to lung transplant survival and is generally resistant to medical therapy. Extracorporeal photophoresis (ECP) is an immunomodulatory therapy that shows promise in stabilizing BOS patients, but its mechanisms of action are unclear. In a mouse lung transplant model, we show that ECP blunts alloimmune responses and inhibits BOS through lowering airway TGF-β bioavailability without altering its expression. Surprisingly, ECP-treated leukocytes were primarily engulfed by alveolar macrophages (AMs), which were reprogrammed to become less responsive to TGF-β and reduce TGF-β bioavailability through secretion of the TGF-β antagonist decorin. In untreated recipients, high airway TGF-β activity stimulated AMs to express CCL2, leading to CCR2+ monocyte-driven BOS development. Moreover, we found TGF-β receptor 2-dependent differentiation of CCR2+ monocytes was required for the generation of monocyte-derived AMs, which in turn promoted BOS by expanding tissue-resident memory CD8+ T cells that inflicted airway injury through Blimp-1-mediated granzyme B expression. Thus, through studying the effects of ECP, we have identified an AM functional plasticity that controls a TGF-β-dependent network that couples CCR2+ monocyte recruitment and differentiation to alloimmunity and BOS.
Keywords: Adaptive immunity; Immunology; Inflammation; Monocytes; Organ transplantation.
Figures


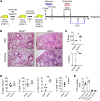

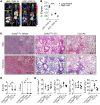


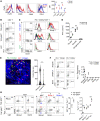
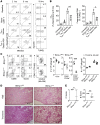
References
-
- Yusen RD, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-third adult lung and heart-lung transplant report-2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant. 2016;35(10):1170–1184. - PubMed
-
- Itabashi Y, et al. Decline in club cell secretory proteins, exosomes induction and immune responses to lung self-antigens, Kα1 tubulin and collagen V, leading to chronic rejection after human lung transplantation. Transplantation. 2021;105(6):1337–1346. doi: 10.1097/TP.0000000000003428. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

