Serial block face scanning electron microscopy reveals region-dependent remodelling of transverse tubules post-myocardial infarction
- PMID: 36189812
- PMCID: PMC9527908
- DOI: 10.1098/rstb.2021.0331
Serial block face scanning electron microscopy reveals region-dependent remodelling of transverse tubules post-myocardial infarction
Abstract
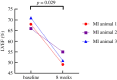
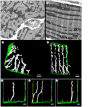
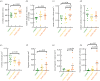
The highly organized transverse tubule (t-tubule) network facilitates cardiac excitation-contraction coupling and synchronous cardiac myocyte contraction. In cardiac failure secondary to myocardial infarction (MI), changes in the structure and organization of t-tubules result in impaired cardiac contractility. However, there is still little knowledge on the regional variation of t-tubule remodelling in cardiac failure post-MI. Here, we investigate post-MI t-tubule remodelling in infarct border and remote regions, using serial block face scanning electron microscopy (SBF-SEM) applied to a translationally relevant sheep ischaemia reperfusion MI model and matched controls. We performed minimally invasive coronary angioplasty of the left anterior descending artery, followed by reperfusion after 90 min to establish the MI model. Left ventricular tissues obtained from control and MI hearts eight weeks post-MI were imaged using SBF-SEM. Image analysis generated three-dimensional reconstructions of the t-tubular network in control, MI border and remote regions. Quantitative analysis revealed that the MI border region was characterized by t-tubule depletion and fragmentation, dilation of surviving t-tubules and t-tubule elongation. This study highlights region-dependent remodelling of the tubular network post-MI and may provide novel localized therapeutic targets aimed at preservation or restoration of the t-tubules to manage cardiac contractility post-MI. This article is part of the theme issue 'The cardiomyocyte: new revelations on the interplay between architecture and function in growth, health, and disease'.
Keywords: cardiomyocyte; myocardial infarction; remodelling; three-dimensional electron microscopy; transverse tubules.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

