Fifty years with aspirin and platelets
- PMID: 36189951
- PMCID: PMC10099789
- DOI: 10.1111/bph.15966
Fifty years with aspirin and platelets
Abstract
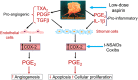
In 2021, we reached the 50th anniversary of the publication of Sir John Vane's seminal paper in Nature New Biology describing the experiments supporting his mechanistic hypothesis that inhibition of prostaglandin synthesis might explain the main pharmacological effects of aspirin and aspirin-like drugs, that is, reduction in pain, fever and inflammation. Bengt Samuelsson's subsequent discoveries elucidating the cyclooxygenase pathway of platelet arachidonic acid metabolism motivated my research interest towards measuring platelet thromboxane A2 biosynthesis as a tool to investigate the clinical pharmacology of cyclooxygenase inhibition by aspirin in health and disease. What followed was a long, winding road of clinical research leading to the characterization of low-dose aspirin as a life-saving antiplatelet drug that still represents the cornerstone of antithrombotic therapy. Having witnessed and participated in these 50 years of aspirin research, I thought of providing a personal testimony of how things developed and eventually led to a remarkable success story of independent research.
Keywords: aspirin; cardiac pharmacology; clinical pharmacology; cyclo-oxygenase; pharmacodynamics; platelets/thrombocytes; prostaglandins.
© 2022 The Author. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
During the past 20 years, I have received grant support for investigator‐initiated research from the Italian Drug Agency (AIFA), Bayer AG, Cancer Research UK and the European Commission, FP6 and FP7 Programmes. During the past 5 years, I have received consultant and speaker fees from Acticor Biotech, Amgen, Bayer, Eli Lilly, GlaxoSmithKline, Tremeau and Zambon. I chair the Scientific Advisory Board of the International Aspirin Foundation.
Figures
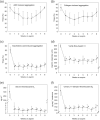

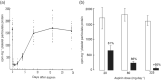
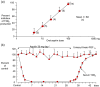


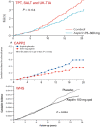
References
-
- Born, G. V. R. (1962a). Quantitative investigations into the aggregation of blood platelets. The Journal of Physiology, 162, 7–68.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

