Graph-Driven Reaction Discovery: Progress, Challenges, and Future Opportunities
- PMID: 36190262
- PMCID: PMC9574932
- DOI: 10.1021/acs.jpca.2c06408
Graph-Driven Reaction Discovery: Progress, Challenges, and Future Opportunities
Abstract
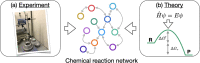
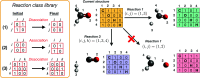
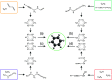
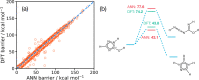
Graph-based descriptors, such as bond-order matrices and adjacency matrices, offer a simple and compact way of categorizing molecular structures; furthermore, such descriptors can be readily used to catalog chemical reactions (i.e., bond-making and -breaking). As such, a number of graph-based methodologies have been developed with the goal of automating the process of generating chemical reaction network models describing the possible mechanistic chemistry in a given set of reactant species. Here, we outline the evolution of these graph-based reaction discovery schemes, with particular emphasis on more recent methods incorporating graph-based methods with semiempirical and ab initio electronic structure calculations, minimum-energy path refinements, and transition state searches. Using representative examples from homogeneous catalysis and interstellar chemistry, we highlight how these schemes increasingly act as "virtual reaction vessels" for interrogating mechanistic questions. Finally, we highlight where challenges remain, including issues of chemical accuracy and calculation speeds, as well as the inherent challenge of dealing with the vast size of accessible chemical reaction space.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Angeli D. A Tutorial on Chemical Reaction Network Dynamics. Eur. J. Control 2009, 15, 398–406. 10.3166/ejc.15.398-406. - DOI
-
- Wakelam V.; Smith I. W. M.; Herbst E.; Troe J.; Geppert W.; Linnartz H.; Oberg K.; Roueff E.; Agundez M.; Pernot P.; et al. Reaction Networks For Interstellar Chemical Modelling: Improvements and Challenges. Space Sci. Rev. 2010, 156, 13–72. 10.1007/s11214-010-9712-5. - DOI
-
- Rangarajan S.; Brydon R. R. O.; Bhan A.; Daoutidis P. Automated identification of energetically feasible mechanisms of complex reaction networks in heterogeneous catalysis: application to glycerol conversion on transition metals. Green Chem. 2014, 16, 813–823. 10.1039/C3GC41386A. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

