Non-trough adalimumab and certolizumab drug levels associated with a therapeutic EULAR response in adherent patients with rheumatoid arthritis
- PMID: 36190343
- PMCID: PMC10234202
- DOI: 10.1093/rheumatology/keac564
Non-trough adalimumab and certolizumab drug levels associated with a therapeutic EULAR response in adherent patients with rheumatoid arthritis
Abstract
Objectives: Interventions aimed at increasing TNF-α inhibitor serum drug levels (SDLs) may improve treatment response; however, previous studies suggesting SDL cut-offs have not accounted for treatment adherence. The aim of this study was to establish the relationship between adalimumab/certolizumab SDLs and EULAR good vs non-/moderate response and to define SDL cut-offs associated with good response in fully adherent patients.
Methods: In a prospective observational study, 475 patients with RA were treated with certolizumab (n = 192) or adalimumab (n = 283). At baseline and 3, 6 and 12 months, patients had 28-joint DAS, self-reported treatment adherence and SDLs measured. Fully adherent patients were analysed as a subgroup. Follow-up data at 3, 6 and 12 months were analysed separately. Median SDLs were compared in good vs non-/moderate response patients and receiver operating characteristics (ROC) curves were used to establish cut-off SDLs.
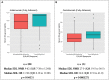
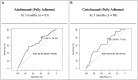
Results: Fully adherent good responders had significantly higher median adalimumab/certolizumab SDLs compared with non-/moderate responders (P = 0.04 and P = 0.0005, respectively). ROC analysis reported 3 month non-trough adalimumab SDLs discriminated good vs non-/moderate response with an area under the curve (AUC) of 0.63 (95% CI 0.52, 0.75), with a cut-off of 7.5 mg/l being 39.1% specific and 80.9% sensitive. Similarly, 3 month non-trough certolizumab SDLs discriminated good vs non-/moderate response with an AUC of 0.65 (95% CI 0.51, 0.78), with a cut-off of 26.0 mg/l being 43.9% specific and 77.8% sensitive.
Conclusion: In fully adherent patients, higher SDLs are detected in good responders, suggesting that interventions to improve SDLs, such as encouraging adherence, could improve treatment response. The 3 month non-trough SDL cut-offs of 7.5 mg/l for adalimumab and 26.0 mg/l for certolizumab may be useful in clinical practice.
Keywords: RA; TNF-α; adalimumab; adherence; certolizumab; inhibitors; serum drug levels; therapeutic response.
© The Author(s) 2022. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures

References
-
- National Collaborating Centre for Chronic Conditions. Rheumatoid arthritis: national clinical guideline for management and treatment in adults. London: Royal College of Physicians, 2009. - PubMed
-
- Kristensen LE, Christensen R, Bliddal H. et al. The number needed to treat for adalimumab, etanercept, and infliximab based on ACR50 response in three randomized controlled trials on established rheumatoid arthritis: a systematic literature review. Scand J Rheumatol 2007;36:411–7. - PubMed
-
- Smolen JS, Landewe R, Bijlsma J. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. - PubMed
-
- John KJ, Sanchez HN, Schoenbrunner N.. Defining response to TNF-inhibitors in rheumatoid arthritis: the negative impact of anti-TNF cycling and the need for a personalized medicine approach to identify primary non-responders. Clin Rheumatol 2019;38:2967–76. - PubMed

