Advancements in antimicrobial nanoscale materials and self-assembling systems
- PMID: 36190355
- PMCID: PMC9575517
- DOI: 10.1039/d1cs00915j
Advancements in antimicrobial nanoscale materials and self-assembling systems
Abstract
Antimicrobial resistance is directly responsible for more deaths per year than either HIV/AIDS or malaria and is predicted to incur a cumulative societal financial burden of at least $100 trillion between 2014 and 2050. Already heralded as one of the greatest threats to human health, the onset of the coronavirus pandemic has accelerated the prevalence of antimicrobial resistant bacterial infections due to factors including increased global antibiotic/antimicrobial use. Thus an urgent need for novel therapeutics to combat what some have termed the 'silent pandemic' is evident. This review acts as a repository of research and an overview of the novel therapeutic strategies being developed to overcome antimicrobial resistance, with a focus on self-assembling systems and nanoscale materials. The fundamental mechanisms of action, as well as the key advantages and disadvantages of each system are discussed, and attention is drawn to key examples within each field. As a result, this review provides a guide to the further design and development of antimicrobial systems, and outlines the interdisciplinary techniques required to translate this fundamental research towards the clinic.
Conflict of interest statement
There are no conflicts to declare.
Figures












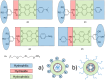

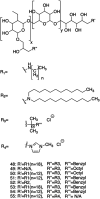

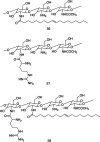



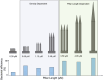


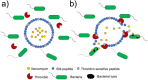
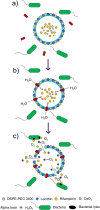
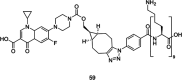

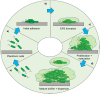









References
-
- Charani E. McKee M. Ahmad R. Balasegaram M. Bonaconsa C. Merrett G. B. Busse R. Carter V. Castro-Sanchez E. Franklin B. D. Georgiou P. Hill-Cawthorne K. Hope W. Imanaka Y. Kambugu A. Leather A. J. M. Mbamalu O. McLeod M. Mendelson M. Mpundu M. Rawson T. M. Ricciardi W. Rodriguez-Manzano J. Singh S. Tsioutis C. Uchea C. Zhu N. N. Holmes A. H. Lancet Reg. Health Europe. 2021;7:100161. doi: 10.1016/j.lanepe.2021.100161. - DOI - PMC - PubMed
-
- O'Neill J., Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance, Wellcome Trust and HM Government, 2016
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

