Sugar Modification of Wall Teichoic Acids Determines Serotype-Dependent Strong Biofilm Production in Listeria monocytogenes
- PMID: 36190419
- PMCID: PMC9603678
- DOI: 10.1128/spectrum.02769-22
Sugar Modification of Wall Teichoic Acids Determines Serotype-Dependent Strong Biofilm Production in Listeria monocytogenes
Abstract
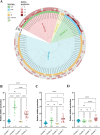
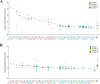
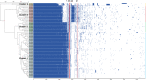
Biofilm production is responsible for persistent food contamination by Listeria monocytogenes, threatening food safety and public health. Human infection and food contamination with L. monocytogenes are caused primarily by serotypes 1/2a, 1/2b, and 4b. However, the association of biofilm production with phylogenic lineage and serotype has not yet been fully understood. In this study, we measured the levels of biofilm production in 98 clinical strains of L. monocytogenes at 37°C, 25°C, and 4°C. The phylogenetic clusters grouped by core genome multilocus sequence typing (cgMLST) exhibited association between biofilm production and phylogenetic lineage and serotype. Whereas clusters 1 and 3 consisting of serotype 4b strains exhibited weak biofilm production, clusters 2 (serotype 1/2b) and 4 (serotype 1/2a) were composed of strong biofilm formers. Particularly, cluster 2 (serotype 1/2b) strains exhibited the highest levels of biofilm production at 37°C, and the levels of biofilm production of cluster 4 (serotype 1/2a) strains were significantly elevated at all tested temperatures. Pan-genome analysis identified 22 genes unique to strong biofilm producers, most of which are related to the synthesis and modification of teichoic acids. Notably, a knockout mutation of the rml genes related to the modification of wall teichoic acids with l-rhamnose, which is specific to serogroup 1/2, significantly reduced the level of biofilm production by preventing biofilm maturation. Here, the results of our study show that biofilm production in L. monocytogenes is related to phylogeny and serotype and that the modification of wall teichoic acids with l-rhamnose is responsible for serotype-specific strong biofilm formation in L. monocytogenes. IMPORTANCE Biofilm formation on the surface of foods or food-processing facilities by L. monocytogenes is a serious food safety concern. Here, our data demonstrate that the level of biofilm production differs among serotypes 1/2a, 1/2b, and 4b depending on the temperature. Furthermore, sugar decoration of bacterial cell walls with l-rhamnose is responsible for strong biofilm production in serotypes 1/2a and 1/2b, commonly isolated from foods and listeriosis cases. The findings in this study improve our understanding of the association of biofilm production with phylogenetic lineage and serotype in L. monocytogenes.
Keywords: Listeria; biofilms; cell wall; rhamnosylation; serotype.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Barton Behravesh C, Jones TF, Vugia DJ, Long C, Marcus R, Smith K, Thomas S, Zansky S, Fullerton KE, Henao OL, Scallan E, FoodNet Working Group . 2011. Deaths associated with bacterial pathogens transmitted commonly through food: Foodborne Diseases Active Surveillance Network (FoodNet), 1996–2005. J Infect Dis 204:263–267. doi:10.1093/infdis/jir263. - DOI - PubMed
-
- Van Walle I, Björkman JT, Cormican M, Dallman T, Mossong J, Moura A, Pietzka A, Ruppitsch W, Takkinen J. 2018. Retrospective validation of whole genome sequencing-enhanced surveillance of listeriosis in Europe, 2010 to 2015. Euro Surveill 23:1700798. doi:10.2807/1560-7917.Es.2018.23.33.1700798. - DOI - PMC - PubMed
-
- Maury MM, Tsai YH, Charlier C, Touchon M, Chenal-Francisque V, Leclercq A, Criscuolo A, Gaultier C, Roussel S, Brisabois A, Disson O, Rocha EPC, Brisse S, Lecuit M. 2016. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat Genet 48:308–313. doi:10.1038/ng.3501. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

