Nanocarriers for cancer nano-immunotherapy
- PMID: 36190661
- PMCID: PMC9528883
- DOI: 10.1007/s13346-022-01241-3
Nanocarriers for cancer nano-immunotherapy
Abstract
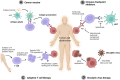
The host immune system possesses an intrinsic ability to target and kill cancer cells in a specific and adaptable manner that can be further enhanced by cancer immunotherapy, which aims to train the immune system to boost the antitumor immune response. Several different categories of cancer immunotherapy have emerged as new standard cancer therapies in the clinic, including cancer vaccines, immune checkpoint inhibitors, adoptive T cell therapy, and oncolytic virus therapy. Despite the remarkable survival benefit for a subset of patients, the low response rate and immunotoxicity remain the major challenges for current cancer immunotherapy. Over the last few decades, nanomedicine has been intensively investigated with great enthusiasm, leading to marked advancements in nanoparticle platforms and nanoengineering technology. Advances in nanomedicine and immunotherapy have also led to the emergence of a nascent research field of nano-immunotherapy, which aims to realize the full therapeutic potential of immunotherapy with the aid of nanomedicine. In particular, nanocarriers present an exciting opportunity in immuno-oncology to boost the activity, increase specificity, decrease toxicity, and sustain the antitumor efficacy of immunological agents by potentiating immunostimulatory activity and favorably modulating pharmacological properties. This review discusses the potential of nanocarriers for cancer immunotherapy and introduces preclinical studies designed to improve clinical cancer immunotherapy modalities using nanocarrier-based engineering approaches. It also discusses the potential of nanocarriers to address the challenges currently faced by immuno-oncology as well as the challenges for their translation to clinical applications.
Keywords: Cancer; Delivery; Immunotherapy; Nano-immunotherapy; Nanomedicine; Nanoparticle.
© 2022. Controlled Release Society.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Fitzmaurice C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–1768. doi: 10.1001/jamaoncol.2019.2996. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

