What are the impacts of increasing cost-effectiveness Threshold? a protocol on an empirical study based on economic evaluations conducted in Thailand
- PMID: 36191016
- PMCID: PMC9529087
- DOI: 10.1371/journal.pone.0274944
What are the impacts of increasing cost-effectiveness Threshold? a protocol on an empirical study based on economic evaluations conducted in Thailand
Abstract
Background: Economic evaluations have been widely used to inform and guide policy-making process in healthcare resources allocation as a part of an evidence package. An intervention is considered cost-effective if an ICER is less than a cost-effectiveness threshold (CET), where a CET represents the acceptable price for a unit of additional health gain which a decision-maker is willing to pay. There has been discussion to increase a CET in many settings such as the United Kingdom and Thailand. To the best of our knowledge, Thailand is the only country that has an explicit CET and has revised their CET, not once but twice. Hence, the situation in Thailand provides a unique opportunity for evaluating the impact of changing CET on healthcare expenditure and manufacturers' behaviours in the real-world setting. Before we decide whether a CET should be increased, information on what happened after the CET was increased in the past could be informative and helpful.
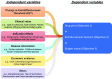
Objectives: This study protocol describes a proposed plan to investigate the impact of increased cost-effectiveness threshold using Thailand as a case study. Specifically, we will examine the impact of increasing CET on the drug prices submitted by pharmaceutical companies to the National List of Essential Medicine (NLEM), the decision to include or exclude medications in the NLEM, and the overall budget impact.
Materials and designs: Retrospective data analysis of the impact of increased CET on national drug committee decisions in Thailand (an upper middle-income country) will be conducted and included data from various sources such as literature, local organizations (e.g. Thai Food and Drug Administration), and inputs from stakeholder consultation meetings. The outcomes include: (1) drug price submitted by the manufacturers and final drug price included in the NLEM if available; (2) decisions about whether the drug was included in the NLEM for reimbursement; and (3) budget impact. The independent variables include a CET, the variable of interest, which can take values of THB100,000, THB120,000, or THB160,000, and potential confounders such as whether this drug was for a chronic disease, market size, and primary endpoint. We will conduct separate multivariable regression analysis for each outcome specified above.
Discussion: Understanding the impact of increasing the CET would be helpful in assisting the decision to use and develop an appropriate threshold for one's own setting. Due to the nature of the study design, the findings will be prone to confounding effect and biases; therefore, the analyses will be adjusted for potential confounders and statistical methods will be explored to minimize biases. Knowledge gained from the study will be conveyed to the public through various disseminations such as reports, policy briefs, academic journals, and presentations.
Conflict of interest statement
This work is funded by the Health Systems Research Institute (HSRI) of Thailand (https://www.hsri.or.th), Grant number HSRI. 64-159. The funders have no contribution to the study such as the study design, data collection, and data analysis. HITAP is funded by national and international public funding agencies. iDSI is funded by the Bill & Melinda Gates Foundation (OPP1202541), the UK’s Department for International Development, and the Rockefeller Foundation. HITAP is also supported by the Access and Delivery Partnership, which is hosted by the United Nations Development Programme and funded by the Government of Japan.
Figures
Similar articles
-
Cost-effectiveness and budget impact analysis of infliximab and its biosimilar in patients with refractory moderate-to-severe Crohn's disease using real world evidence in Thailand.J Med Econ. 2020 Nov;23(11):1302-1310. doi: 10.1080/13696998.2020.1803889. Epub 2020 Aug 13. J Med Econ. 2020. PMID: 32729347
-
Cost-effectiveness and budget impact analyses of colorectal cancer screenings in a low- and middle-income country: example from Thailand.J Med Econ. 2019 Dec;22(12):1351-1361. doi: 10.1080/13696998.2019.1674065. Epub 2019 Oct 12. J Med Econ. 2019. PMID: 31560247
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Factors associated with the author-reported cost-effectiveness threshold in high-income countries: systematic review and multivariable modelling.Eur J Health Econ. 2024 Jun;25(4):631-639. doi: 10.1007/s10198-023-01613-7. Epub 2023 Jul 11. Eur J Health Econ. 2024. PMID: 37433889
-
Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices--budget impact analysis.Value Health. 2007 Sep-Oct;10(5):336-47. doi: 10.1111/j.1524-4733.2007.00187.x. Value Health. 2007. PMID: 17888098
Cited by
-
Establishing cost-effectiveness threshold in China: a community survey of willingness to pay for a healthylife year.BMJ Glob Health. 2024 Jan 9;9(1):e013070. doi: 10.1136/bmjgh-2023-013070. BMJ Glob Health. 2024. PMID: 38195152 Free PMC article.
-
Cost-effectiveness of tenofovir prophylaxis during pregnancy for the elimination of mother-to-child transmission of the hepatitis B virus: real-world analysis from Thailand.BMJ Open. 2023 Jul 19;13(7):e067275. doi: 10.1136/bmjopen-2022-067275. BMJ Open. 2023. PMID: 37474179 Free PMC article.
-
Health impact and cost-effectiveness of vaccination using potential next-generation influenza vaccines in Thailand: a modelling study.BMJ Glob Health. 2024 Nov 18;9(11):e015837. doi: 10.1136/bmjgh-2024-015837. BMJ Glob Health. 2024. PMID: 39557448 Free PMC article.
-
Use of Cost-Effectiveness Thresholds in Healthcare Public Policy: Progress and Challenges.Appl Health Econ Health Policy. 2024 Nov;22(6):797-804. doi: 10.1007/s40258-024-00900-5. Epub 2024 Jul 12. Appl Health Econ Health Policy. 2024. PMID: 38995492 Free PMC article. Review.
References
-
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes: Oxford university press; 2015.
-
- Medical Association of Thailand. Journal of the Medical Association of Thailand. 2014;97(5).
-
- Canadian Agency for Drugs and Technologies in Health. Guidelines for the Economic Evaluation of Health Technologies: Canada. 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous