Human neutrophil IL1β directs intestinal epithelial cell extrusion during Salmonella infection
- PMID: 36191054
- PMCID: PMC9578578
- DOI: 10.1371/journal.ppat.1010855
Human neutrophil IL1β directs intestinal epithelial cell extrusion during Salmonella infection
Abstract
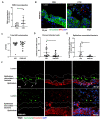
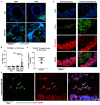
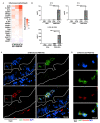
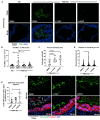
Infection of the human gut by Salmonella enterica Typhimurium (STM) results in a localized inflammatory disease that is not mimicked in murine infections. To determine mechanisms by which neutrophils, as early responders to bacterial challenge, direct inflammatory programming of human intestinal epithelium, we established a multi-component human intestinal organoid (HIO) model of STM infection. HIOs were micro-injected with STM and seeded with primary human polymorphonuclear leukocytes (PMN-HIOs). PMNs did not significantly alter luminal colonization of Salmonella, but their presence reduced intraepithelial bacterial burden. Adding PMNs to infected HIOs resulted in substantial accumulation of shed TUNEL+ epithelial cells that was driven by PMN Caspase-1 activity. Inhibition of Caspases-1, -3 or -4 abrogated epithelial cell death and extrusion in the infected PMN-HIOs but only Caspase-1 inhibition significantly increased bacterial burden in the PMN-HIO epithelium. Thus, PMNs promote cell death in human intestinal epithelial cells through multiple caspases as a protective response to infection. IL-1β was necessary and sufficient to induce cell shedding in the infected HIOs. These data support a critical innate immune function for human neutrophils in amplifying cell death and extrusion of human epithelial cells from the Salmonella-infected intestinal monolayer.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Salmonella Homepage. 27 Jul 2022 [cited 27 Jul 2022]. Available: https://www.cdc.gov/salmonella/index.html
-
- Sellin ME, Müller AA, Felmy B, Dolowschiak T, Diard M, Tardivel A, et al.. Epithelium-intrinsic NAIP/NLRC4 inflammasome drives infected enterocyte expulsion to restrict Salmonella replication in the intestinal mucosa. Cell Host Microbe. 2014;16: 237–248. doi: 10.1016/j.chom.2014.07.001 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

