A Prospective Study of Lumbar Facet Arthroplasty in the Treatment of Degenerative Spondylolisthesis and Stenosis: Results from the Total Posterior Spine System (TOPS) IDE Study
- PMID: 36191093
- PMCID: PMC9949521
- DOI: 10.1097/BSD.0000000000001365
A Prospective Study of Lumbar Facet Arthroplasty in the Treatment of Degenerative Spondylolisthesis and Stenosis: Results from the Total Posterior Spine System (TOPS) IDE Study
Abstract
Study design: Prospective randomized Food and Drug Administration investigational device exemption clinical trial.
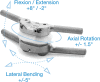
Objective: The purpose of the present study is to report the 1-year clinical and radiographic outcomes and safety profile of patients who underwent lumbar facet arthroplasty through implantation of the Total Posterior Spine System (TOPS) device.
Summary of background data: Lumbar facet arthroplasty is one proposed method of dynamic stabilization to treat grade-1 spondylolisthesis with stenosis; however, there are currently no Food and Drug Administration-approved devices for facet arthroplasty.
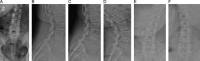
Methods: Standard demographic information was collected for each patient. Radiographic parameters and patient-reported outcome measures were assessed preoperatively and at regular postoperative intervals. Complication and reoperation data were also collected for each patient.
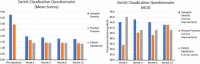
Results: At the time of this study, 153 patients had undergone implantation of the TOPS device. The mean surgical time was 187.8 minutes and the mean estimated blood loss was 205.7cc. The mean length of hospital stay was 3.0 days. Mean Oswestry Disability Index, Visual Analog Score leg and back, and Zurich Claudication Questionnaire scores improved significantly at all postoperative time points ( P >0.001). There were no clinically significant changes in radiographic parameters, and all operative segments remained mobile at 1-year follow-up. Postoperative complications occurred in 11 patients out of the 153 patients (7.2%) who underwent implantation of the TOPS device. Nine patients (5.9%) underwent a total of 13 reoperations, 1 (0.6%) of which was for device-related failure owing to bilateral L5 pedicle screw loosening.
Conclusions: Lumbar facet arthroplasty with the TOPS device demonstrated a statistically significant improvement in all patient-reported outcome measures and the ability to maintain motion at the index level while limiting sagittal translation with a low complication rate.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

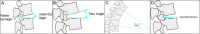




References
-
- Martin BI, Mirza SK, Spina N, et al. . Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine. 2019;44:369–376. - PubMed
-
- Cheh G, Bridwell KH, Lenke LG, et al. . Adjacent segment disease followinglumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine. 2007;32:2253–2257. - PubMed
-
- Eck JC, Humphreys SC, Hodges SD. Adjacent-segment degeneration after lumbar fusion: a review of clinical, biomechanical, and radiologic studies. Am J Orthop. 1999;28:336–340. - PubMed
-
- Kim TH, Lee BH, Moon SH, et al. . Comparison of adjacent segment degeneration after successful posterolateral fusion with unilateral or bilateral pedicle screw instrumentation: a minimum 10-year follow-up. Spine J. 2013;13:1208–1216. - PubMed
-
- Schlegel JD, Smith JA, Schleusener RL. Lumbar motion segment pathology adjacent to thoracolumbar, lumbar, and lumbosacral fusions. Spine. 1996;21:970–981. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

