Preclinical studies on the use of a P-selectin-blocking monoclonal antibody to halt progression of myelofibrosis in the Gata1low mouse model
- PMID: 36191885
- PMCID: PMC10450205
- DOI: 10.1016/j.exphem.2022.09.004
Preclinical studies on the use of a P-selectin-blocking monoclonal antibody to halt progression of myelofibrosis in the Gata1low mouse model
Abstract
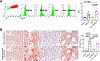
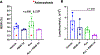
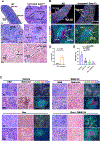
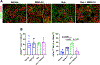
The bone marrow (BM) and spleen from patients with myelofibrosis (MF), as well as those from the Gata1low mouse model of the disease contain increased number of abnormal megakaryocytes. These cells express high levels of the adhesion receptor P-selectin on their surface, which triggers a pathologic neutrophil emperipolesis, leading to increased bioavailability of transforming growth factor-β (TGF-β) in the microenvironment and disease progression. With age, Gata1low mice develop a phenotype similar to that of patients with MF, which is the most severe of the Philadelphia-negative myeloproliferative neoplasms. We previously demonstrated that Gata1low mice lacking the P-selectin gene do not develop MF. In the current study, we tested the hypothesis that pharmacologic inhibition of P-selectin may normalize the phenotype of Gata1low mice that have already developed MF. To test this hypothesis, we have investigated the phenotype expressed by aged Gata1low mice treated with the antimouse monoclonal antibody RB40.34, alone and also in combination with ruxolitinib. The results indicated that RB40.34 in combination with ruxolitinib normalizes the phenotype of Gata1low mice with limited toxicity by reducing fibrosis and the content of TGF-β and CXCL1 (two drivers of fibrosis in this model) in the BM and spleen and by restoring hematopoiesis in the BM and the architecture of the spleen. In conclusion, we provide preclinical evidence that treatment with an antibody against P-selectin in combination with ruxolitinib may be more effective than ruxolitinib alone to treat MF in patients.
Copyright © 2022 ISEH -- Society for Hematology and Stem Cells. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest
PV, FG, FM, MF, AV, GS and MZ declare no conflict. CW, AB and AP are employee of Novartis Pharmaceutical Corporation. ARM received research funds from Novartis Pharmaceutical Corporation.
Figures






Similar articles
-
The Variation in the Traits Ameliorated by Inhibitors of JAK1/2, TGF-β, P-Selectin, and CXCR1/CXCR2 in the Gata1low Model Suggests That Myelofibrosis Should Be Treated by These Drugs in Combination.Int J Mol Sci. 2024 Jul 13;25(14):7703. doi: 10.3390/ijms25147703. Int J Mol Sci. 2024. PMID: 39062946 Free PMC article.
-
P-Selectin Sustains Extramedullary Hematopoiesis in the Gata1 low Model of Myelofibrosis.Stem Cells. 2016 Jan;34(1):67-82. doi: 10.1002/stem.2229. Epub 2015 Oct 23. Stem Cells. 2016. PMID: 26439305
-
Novel targets to cure primary myelofibrosis from studies on Gata1low mice.IUBMB Life. 2020 Jan;72(1):131-141. doi: 10.1002/iub.2198. Epub 2019 Nov 21. IUBMB Life. 2020. PMID: 31749302 Free PMC article. Review.
-
Inhibition of CXCR1/2 reduces the emperipolesis between neutrophils and megakaryocytes in the Gata1low model of myelofibrosis.Exp Hematol. 2023 May;121:30-37. doi: 10.1016/j.exphem.2023.02.003. Epub 2023 Mar 1. Exp Hematol. 2023. PMID: 36863479 Free PMC article.
-
Understanding Splenomegaly in Myelofibrosis: Association with Molecular Pathogenesis.Int J Mol Sci. 2018 Mar 18;19(3):898. doi: 10.3390/ijms19030898. Int J Mol Sci. 2018. PMID: 29562644 Free PMC article. Review.
Cited by
-
CXCL8 and its cognate receptors CXCR1/CXCR2 in primary myelofibrosis.Haematologica. 2024 Jul 1;109(7):2060-2072. doi: 10.3324/haematol.2023.284921. Haematologica. 2024. PMID: 38426279 Free PMC article. Review.
-
The Variation in the Traits Ameliorated by Inhibitors of JAK1/2, TGF-β, P-Selectin, and CXCR1/CXCR2 in the Gata1low Model Suggests That Myelofibrosis Should Be Treated by These Drugs in Combination.Int J Mol Sci. 2024 Jul 13;25(14):7703. doi: 10.3390/ijms25147703. Int J Mol Sci. 2024. PMID: 39062946 Free PMC article.
-
Recent advances in therapies for primary myelofibrosis.Fac Rev. 2023 Sep 26;12:23. doi: 10.12703/r/12-23. eCollection 2023. Fac Rev. 2023. PMID: 37771602 Free PMC article. Review.
-
Transcription factor genetics and biology in predisposition to bone marrow failure and hematological malignancy.Front Oncol. 2023 Jun 12;13:1183318. doi: 10.3389/fonc.2023.1183318. eCollection 2023. Front Oncol. 2023. PMID: 37377909 Free PMC article. Review.
References
-
- Marcellino BK, Verstovsek S, Mascarenhas J. The Myelodepletive Phenotype in Myelofibrosis: Clinical Relevance and Therapeutic Implication. Clin Lymphoma Myeloma Leuk. 2020;20:415–21. - PubMed
-
- Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene. 2013;32:2601–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

