Systematic review of copper intrauterine contraception continuation in young nulliparous women based on intrauterine device type
- PMID: 36192095
- PMCID: PMC9535170
- DOI: 10.1136/bmjopen-2021-060606
Systematic review of copper intrauterine contraception continuation in young nulliparous women based on intrauterine device type
Abstract
Objectives: No copper intrauterine device (IUD) type is known to better suit young nulliparous women who tend to experience higher rates of IUD discontinuation compared with their older parous counterparts. A systematic review to determine which IUDs have higher continuation rates in young nulliparous women was undertaken.
Design: Systematic review and meta-analyses of available evidence based on IUD type.
Data sources: AMED, BNI, CINAHL, DARE, EMBASE, EMCARE, HMIC, MEDLINE, PsycINFO, PubMed, TRIP, and the Cochrane Library electronic databases were searched from inception to 11 May 2022; as well as the Bandolier, Medicines and Healthcare products Regulatory Agency, Faculty of Sexual and Reproductive Healthcare, Royal College of Obstetricians and Gynaecologists, Department of Health, National Institute for Health and Care Excellence, Scottish Intercollegiate Guidelines, WHO and Google Scholar websites.
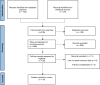
Eligibility criteria: All studies on IUDs currently available in the UK or comparable (same design and size) to those available in the UK, involving nulliparous women of any age including those aged under 30.
Data extraction and synthesis: Independently extracted data were assessed as low risk of bias using the Mixed Methods Appraisal Tool. Random effects meta-analyses of proportions were performed where data, including subgroups, were amenable to quantitative synthesis. Heterogeneity was reported using tau2 and I2 statistics, and sensitivity analyses were also performed.
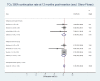
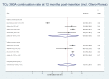
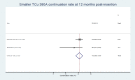
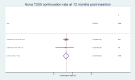
Results: Nineteen studies involving 13 045 nulliparous women were included but the heterogeneity of participant ages, parity and IUD types made quantitative synthesis of outcome data in totality inappropriate. The highest continuation rate obtained was 91.02% (95% CI 88.01% to 93.64%) for the smaller TCu 380A at 12 months post insertion.
Conclusions: Evidence for IUD use in young nulliparous women based on IUD type remains limited. Smaller sized IUD types appear better suited to this group of IUD users, however, more research is needed.
Prospero registration number: CRD42019120969.
Keywords: community gynaecology; public health; reproductive medicine.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Similar articles
-
Menstrual characteristics and ultrasonographic uterine cavity measurements predict bleeding and pain in nulligravid women using intrauterine contraception.Hum Reprod. 2015 Jul;30(7):1580-8. doi: 10.1093/humrep/dev102. Epub 2015 May 19. Hum Reprod. 2015. PMID: 25990577
-
Use of intrauterine devices in nulliparous women.Contraception. 2017 Jun;95(6):529-537. doi: 10.1016/j.contraception.2016.08.011. Epub 2016 Aug 31. Contraception. 2017. PMID: 27591814
-
Twelve-month comparative multicenter study of the TCu 380A and ML 250 intrauterine devices in Bangkok, Thailand.Contraception. 1998 Oct;58(4):201-6. doi: 10.1016/s0010-7824(98)00097-3. Contraception. 1998. PMID: 9865999 Clinical Trial.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
A review of the use of intrauterine devices in nulliparous women.Contraception. 1982 Oct;26(4):323-46. doi: 10.1016/0010-7824(82)90101-9. Contraception. 1982. PMID: 6759026 Review.
References
-
- Department of Health & Social Care: National Statistics . Abortion statistics, England and Wales: 2020, 2021. Available: https://www.gov.uk/government/statistics/abortion-statistics-for-england... [Accessed 20 Dec 2021].
-
- NHS Digital . Statistics on sexual and reproductive health services (contraception): data (tables 6 and 7), 2021. Available: https://digital.nhs.uk/data-and-information/publications/statistical/sex... [Accessed 23 Dec 2021].
-
- BMJ Group and the Royal Pharmaceutical Society of Great Britain . British National formulary, 2021. Available: https://bnf.nice.org.uk/medicinal-forms/intra-uterine-contraceptive-devi... [Accessed 20 Dec 2021].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
