Differentiating Multiple Sclerosis From AQP4-Neuromyelitis Optica Spectrum Disorder and MOG-Antibody Disease With Imaging
- PMID: 36192175
- PMCID: PMC9869760
- DOI: 10.1212/WNL.0000000000201465
Differentiating Multiple Sclerosis From AQP4-Neuromyelitis Optica Spectrum Disorder and MOG-Antibody Disease With Imaging
Abstract
Background and objectives: Relapsing-remitting multiple sclerosis (RRMS), aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder (AQP4-NMOSD), and myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) may have overlapping clinical features. There is an unmet need for imaging markers that differentiate between them when serologic testing is unavailable or ambiguous. We assessed whether imaging characteristics typical of MS discriminate RRMS from AQP4-NMOSD and MOGAD, alone and in combination.
Methods: Adult, nonacute patients with RRMS, APQ4-NMOSD, and MOGAD and healthy controls were prospectively recruited at the National Hospital for Neurology and Neurosurgery (London, United Kingdom) and the Walton Centre (Liverpool, United Kingdom) between 2014 and 2019. They underwent conventional and advanced brain, cord, and optic nerve MRI and optical coherence tomography (OCT).
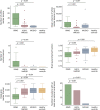
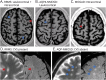
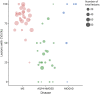
Results: A total of 91 consecutive patients (31 RRMS, 30 APQ4-NMOSD, and 30 MOGAD) and 34 healthy controls were recruited. The most accurate measures differentiating RRMS from AQP4-NMOSD were the proportion of lesions with the central vein sign (CVS) (84% vs 33%, accuracy/specificity/sensitivity: 91/88/93%, p < 0.001), followed by cortical lesions (median: 2 [range: 1-14] vs 1 [0-1], accuracy/specificity/sensitivity: 84/90/77%, p = 0.002) and white matter lesions (mean: 39.07 [±25.8] vs 9.5 [±14], accuracy/specificity/sensitivity: 78/84/73%, p = 0.001). The combination of higher proportion of CVS, cortical lesions, and optic nerve magnetization transfer ratio reached the highest accuracy in distinguishing RRMS from AQP4-NMOSD (accuracy/specificity/sensitivity: 95/92/97%, p < 0.001). The most accurate measures favoring RRMS over MOGAD were white matter lesions (39.07 [±25.8] vs 1 [±2.3], accuracy/specificity/sensitivity: 94/94/93%, p = 0.006), followed by cortical lesions (2 [1-14] vs 1 [0-1], accuracy/specificity/sensitivity: 84/97/71%, p = 0.004), and retinal nerve fiber layer thickness (RNFL) (mean: 87.54 [±13.83] vs 75.54 [±20.33], accuracy/specificity/sensitivity: 80/79/81%, p = 0.009). Higher cortical lesion number combined with higher RNFL thickness best differentiated RRMS from MOGAD (accuracy/specificity/sensitivity: 84/92/77%, p < 0.001).
Discussion: Cortical lesions, CVS, and optic nerve markers achieve a high accuracy in distinguishing RRMS from APQ4-NMOSD and MOGAD. This information may be useful in clinical practice, especially outside the acute phase and when serologic testing is ambiguous or not promptly available.
Classification of evidence: This study provides Class II evidence that selected conventional and advanced brain, cord, and optic nerve MRI and OCT markers distinguish adult patients with RRMS from AQP4-NMOSD and MOGAD.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
