The mitochondrial calcium uniporter of pulmonary type 2 cells determines severity of acute lung injury
- PMID: 36192486
- PMCID: PMC9529882
- DOI: 10.1038/s41467-022-33543-y
The mitochondrial calcium uniporter of pulmonary type 2 cells determines severity of acute lung injury
Abstract
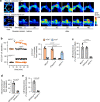
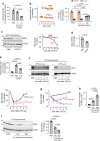
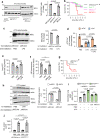
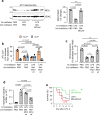
Acute Lung Injury (ALI) due to inhaled pathogens causes high mortality. Underlying mechanisms are inadequately understood. Here, by optical imaging of live mouse lungs we show that a key mechanism is the viability of cytosolic Ca2+ buffering by the mitochondrial Ca2+ uniporter (MCU) in the lung's surfactant-secreting, alveolar type 2 cells (AT2). The buffering increased mitochondrial Ca2+ and induced surfactant secretion in wild-type mice, but not in mice with AT2-specific MCU knockout. In the knockout mice, ALI due to intranasal LPS instillation caused severe pulmonary edema and mortality, which were mitigated by surfactant replenishment prior to LPS instillation, indicating surfactant's protective effect against alveolar edema. In wild-type mice, intranasal LPS, or Pseudomonas aeruginosa decreased AT2 MCU. Loss of MCU abrogated buffering. The resulting mortality was reduced by spontaneous recovery of MCU expression, or by MCU replenishment. Enhancement of AT2 mitochondrial buffering, hence endogenous surfactant secretion, through MCU replenishment might be a therapy against ALI.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Update of
-
The Mitochondrial Calcium Uniporter of Pulmonary Type 2 Cells Determines Severity of ARDS.bioRxiv [Preprint]. 2021 Jan 18:2021.01.18.427173. doi: 10.1101/2021.01.18.427173. bioRxiv. 2021. Update in: Nat Commun. 2022 Oct 3;13(1):5837. doi: 10.1038/s41467-022-33543-y. PMID: 33469582 Free PMC article. Updated. Preprint.
Similar articles
-
The Mitochondrial Calcium Uniporter of Pulmonary Type 2 Cells Determines Severity of ARDS.bioRxiv [Preprint]. 2021 Jan 18:2021.01.18.427173. doi: 10.1101/2021.01.18.427173. bioRxiv. 2021. Update in: Nat Commun. 2022 Oct 3;13(1):5837. doi: 10.1038/s41467-022-33543-y. PMID: 33469582 Free PMC article. Updated. Preprint.
-
Shikonin ameliorated LPS-induced acute lung injury in mice via modulating MCU-mediated mitochondrial Ca2+ and macrophage polarization.Phytomedicine. 2024 Dec;135:156043. doi: 10.1016/j.phymed.2024.156043. Epub 2024 Sep 12. Phytomedicine. 2024. PMID: 39366155
-
HDAC3 deficiency protects against acute lung injury by maintaining epithelial barrier integrity through preserving mitochondrial quality control.Redox Biol. 2023 Jul;63:102746. doi: 10.1016/j.redox.2023.102746. Epub 2023 May 20. Redox Biol. 2023. PMID: 37244125 Free PMC article.
-
Progress in understanding mitochondrial calcium uniporter complex-mediated calcium signalling: A potential target for cancer treatment.Br J Pharmacol. 2019 May;176(9):1190-1205. doi: 10.1111/bph.14632. Epub 2019 Apr 3. Br J Pharmacol. 2019. PMID: 30801705 Free PMC article. Review.
-
Ca2+ Sensors Assemble: Function of the MCU Complex in the Pancreatic Beta Cell.Cells. 2022 Jun 22;11(13):1993. doi: 10.3390/cells11131993. Cells. 2022. PMID: 35805078 Free PMC article. Review.
Cited by
-
Knockdown of hepatic mitochondrial calcium uniporter mitigates MASH and fibrosis in mice.Cell Biosci. 2024 Nov 10;14(1):135. doi: 10.1186/s13578-024-01315-4. Cell Biosci. 2024. PMID: 39523398 Free PMC article.
-
Pulmonary surfactant and prostaglandin E2 in airway smooth muscle relaxation of human and male guinea pigs.Physiol Rep. 2024 Sep;12(17):e70026. doi: 10.14814/phy2.70026. Physiol Rep. 2024. PMID: 39245804 Free PMC article.
-
Possible mechanisms of SARS-CoV-2-associated myocardial fibrosis: reflections in the post-pandemic era.Front Microbiol. 2024 Oct 8;15:1470953. doi: 10.3389/fmicb.2024.1470953. eCollection 2024. Front Microbiol. 2024. PMID: 39444690 Free PMC article. Review.
-
MCU complex: Exploring emerging targets and mechanisms of mitochondrial physiology and pathology.J Adv Res. 2025 Feb;68:271-298. doi: 10.1016/j.jare.2024.02.013. Epub 2024 Feb 27. J Adv Res. 2025. PMID: 38417574 Free PMC article. Review.
-
Macrophage-specific lipid nanoparticle therapy blocks the lung's mechanosensitive immunity due to macrophage-epithelial interactions.bioRxiv [Preprint]. 2024 Oct 26:2023.05.24.541735. doi: 10.1101/2023.05.24.541735. bioRxiv. 2024. PMID: 37292826 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

