The relationship between systemic immune inflammation index and survival in patients with metastatic renal cell carcinomatreated withtyrosine kinase inhibitors
- PMID: 36192500
- PMCID: PMC9529965
- DOI: 10.1038/s41598-022-20056-3
The relationship between systemic immune inflammation index and survival in patients with metastatic renal cell carcinomatreated withtyrosine kinase inhibitors
Abstract
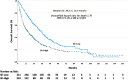
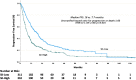
This study aims to investigate the prognostic value of the systemic immune-inflammation index (SII)and its impact on survival in patients with metastatic renal cell carcinoma (mRCC). A total of 706patients with mRCC treated with tyrosine kinase inhibitors (TKIs)between January 2007 and June 2020 (i.e., sunitinib, pazopanib) were included in this study. SII was calculated in 621 patients with the following formula:[neutrophil (cellsx109/L) x platelet (cellsx109/L)] / lymphocyte (cellsx109/L).All patients were classified into SII-high and SII-low groups based on the cut-off value of SII at 756, which was the median SII level of our study group. The minimal follow-up duration was 10 months in all cohorts. The median age of patients was 60 (interquartile range (IQR):53-67) years. Three out of four patients were male. The majority of patients (85.7%) had clear cell histology, and sarcomatoid differentiation was observed in 16.9% of all patients. There were 311 and 310 patients in the SII-low and SII-high groups, respectively. In general, baseline characteristics were similar in each group. However, the rate of patients treated with sunitinib (63.3% vs. 49.0%, p < 0.001) and those who underwent nephrectomy (83.6% vs. 64.2%, p < 0.001) was higher in the SII-low group than in the SII-high group. On the other hand, patients with the IMDC poorrisk (31.6% vs. 8.0%, p < 0.001), those with bone (51.8% vs. 32.2%, p < 0.001) or central nervous system (12.9% vs. 5.8%, p = 0.026) metastasis, and those with Eastern Cooperative Oncology Group(ECOG) 2-4 performance score (28.1% vs.17.7%, p = 0.002) were more common in the SII-high group than in the SII-low group. The median overall survival (OS) was longer in the SII-low group than in the SII-high group (34.6 months vs. 14.5 months, p < 0.001). Similarly, the median progression-free survival (PFS) was longer in the SII-low group than in the SII-high group (18.0 months vs. 7.7 months, p < 0.001).In multivariableanalysis, SII was an independent prognostic factor for OS (hazard ratio (HR):1.39, 95% confidence interval (CI):1.05-1.85, p = 0.01) and PFS (HR:1.60, 95% CI:1.24-2.05, p < 0.001).Pre-treatment level of high SII might be considered a predictor of poor prognosisin patients with mRCC treated with TKIs.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Tannir, N.M., Frontera, O.A., Hammers, H.J., et al. Thirty-month follow-up of the phase III CheckMate 214 trial of first-line nivolumab+ ipilimumab (N+ I) or sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). Am. Soc. Clin. Oncol. (2019).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical