Monitoring autochthonous lung tumors induced by somatic CRISPR gene editing in mice using a secreted luciferase
- PMID: 36192757
- PMCID: PMC9531476
- DOI: 10.1186/s12943-022-01661-2
Monitoring autochthonous lung tumors induced by somatic CRISPR gene editing in mice using a secreted luciferase
Abstract
Background: In vivo gene editing of somatic cells with CRISPR nucleases has facilitated the generation of autochthonous mouse tumors, which are initiated by genetic alterations relevant to the human disease and progress along a natural timeline as in patients. However, the long and variable, orthotopic tumor growth in inner organs requires sophisticated, time-consuming and resource-intensive imaging for longitudinal disease monitoring and impedes the use of autochthonous tumor models for preclinical studies.
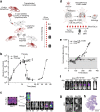
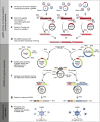
Methods: To facilitate a more widespread use, we have generated a reporter mouse that expresses a Cre-inducible luciferase from Gaussia princeps (GLuc), which is secreted by cells in an energy-consuming process and can be measured quantitatively in the blood as a marker for the viable tumor load. In addition, we have developed a flexible, complementary toolkit to rapidly assemble recombinant adenoviruses (AVs) for delivering Cre recombinase together with CRISPR nucleases targeting cancer driver genes.
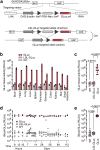
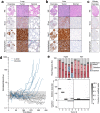
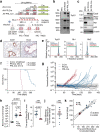
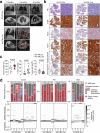
Results: We demonstrate that intratracheal infection of GLuc reporter mice with CRISPR-AVs efficiently induces lung tumors driven by mutations in the targeted cancer genes and simultaneously activates the GLuc transgene, resulting in GLuc secretion into the blood by the growing tumor. GLuc blood levels are easily and robustly quantified in small-volume blood samples with inexpensive equipment, enable tumor detection already several months before the humane study endpoint and precisely mirror the kinetics of tumor development specified by the inducing gene combination.
Conclusions: Our study establishes blood-based GLuc monitoring as an inexpensive, rapid, high-throughput and animal-friendly method to longitudinally monitor autochthonous tumor growth in preclinical studies.
Keywords: Adenovirus; Autochthonous mouse tumor; CRISPR; Luciferase; Lung cancer; Orthotopic tumor.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

