Evaluation of inhaled nitric oxide (iNO) treatment for moderate-to-severe ARDS in critically ill patients with COVID-19: a multicenter cohort study
- PMID: 36192801
- PMCID: PMC9527729
- DOI: 10.1186/s13054-022-04158-y
Evaluation of inhaled nitric oxide (iNO) treatment for moderate-to-severe ARDS in critically ill patients with COVID-19: a multicenter cohort study
Abstract
Background: Inhaled nitric oxide (iNO) is used as rescue therapy in patients with refractory hypoxemia due to severe COVID-19 acute respiratory distress syndrome (ARDS) despite the recommendation against the use of this treatment. To date, the effect of iNO on the clinical outcomes of critically ill COVID-19 patients with moderate-to-severe ARDS remains arguable. Therefore, this study aimed to evaluate the use of iNO in critically ill COVID-19 patients with moderate-to-severe ARDS.
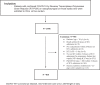
Methods: This multicenter, retrospective cohort study included critically ill adult patients with confirmed COVID-19 treated from March 01, 2020, until July 31, 2021. Eligible patients with moderate-to-severe ARDS were subsequently categorized into two groups based on inhaled nitric oxide (iNO) use throughout their ICU stay. The primary endpoint was the improvement in oxygenation parameters 24 h after iNO use. Other outcomes were considered secondary. Propensity score matching (1:2) was used based on the predefined criteria.
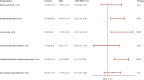
Results: A total of 1598 patients were screened, and 815 were included based on the eligibility criteria. Among them, 210 patients were matched based on predefined criteria. Oxygenation parameters (PaO2, FiO2 requirement, P/F ratio, oxygenation index) were significantly improved 24 h after iNO administration within a median of six days of ICU admission. However, the risk of 30-day and in-hospital mortality were found to be similar between the two groups (HR: 1.18; 95% CI: 0.77, 1.82; p = 0.45 and HR: 1.40; 95% CI: 0.94, 2.11; p= 0.10, respectively). On the other hand, ventilator-free days (VFDs) were significantly fewer, and ICU and hospital LOS were significantly longer in the iNO group. In addition, patients who received iNO had higher odds of acute kidney injury (AKI) (OR (95% CI): 2.35 (1.30, 4.26), p value = 0.005) and hospital/ventilator-acquired pneumonia (OR (95% CI): 3.2 (1.76, 5.83), p value = 0.001).
Conclusion: In critically ill COVID-19 patients with moderate-to-severe ARDS, iNO rescue therapy is associated with improved oxygenation parameters but no mortality benefits. Moreover, iNO use is associated with higher odds of AKI, pneumonia, longer LOS, and fewer VFDs.
Keywords: 30-day mortality; Acute respiratory distress syndrome (ARDS); COVID-19; Critically ill; In-hospital mortality; Inhaled nitric oxide; Intensive care units (ICUs); Oxygenation parameter; SARS-CoV-2; iNO.
© 2022. The Author(s).
Conflict of interest statement
No author has a conflict of interest in this study.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous