Chicken intestinal microbiota modulation of resistance to nephropathogenic infectious bronchitis virus infection through IFN-I
- PMID: 36192807
- PMCID: PMC9527382
- DOI: 10.1186/s40168-022-01348-2
Chicken intestinal microbiota modulation of resistance to nephropathogenic infectious bronchitis virus infection through IFN-I
Abstract
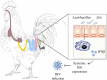
Background: Mammalian intestinal microbiomes are necessary for antagonizing systemic viral infections. However, very few studies have identified whether poultry commensal bacteria play a crucial role in protecting against systemic viral infections. Nephropathogenic infectious bronchitis virus (IBV) is a pathogenic coronavirus that causes high morbidity and multiorgan infection tropism in chickens.
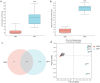
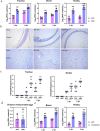
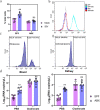
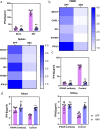
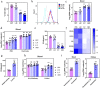
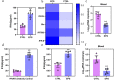
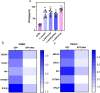
Results: In this study, we used broad-spectrum oral antibiotics (ABX) to treat specific pathogen free (SPF) chickens to deplete the microbiota before infection with nephropathogenic IBV to analyze the impact of microbiota on IBV infections in vivo. Depletion of the SPF chicken microbiota increases pathogenicity and viral burden following IBV infection. The gnotobiotic chicken infection model further demonstrated that intestinal microbes are resistant to nephropathogenic IBV infection. In addition, ABX-treated chickens showed a severe reduction in macrophage activation, impaired type I IFN production, and IFN-stimulated gene expression in peripheral blood mononuclear cells and the spleen. Lactobacillus isolated from SPF chickens could restore microbiota-depleted chicken macrophage activation and the IFNAR-dependent type I IFN response to limit IBV infection. Furthermore, exopolysaccharide metabolites of Lactobacillus spp. could induce IFN-β.
Conclusions: This study revealed the resistance mechanism of SPF chicken intestinal microbiota to nephropathogenic IBV infection, providing new ideas for preventing and controlling nephropathogenic IBV. Video abstract.
Keywords: IFN; Intestinal microbiota; Lactobacillus; Nephropathogenic infectious bronchitis virus; SPF chicken.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Comparative transcriptome analysis reveals induction of apoptosis in chicken kidney cells associated with the virulence of nephropathogenic infectious bronchitis virus.Microb Pathog. 2017 Dec;113:451-459. doi: 10.1016/j.micpath.2017.11.031. Epub 2017 Nov 21. Microb Pathog. 2017. PMID: 29174688 Free PMC article.
-
The Intestinal Microbiome Primes Host Innate Immunity against Enteric Virus Systemic Infection through Type I Interferon.mBio. 2021 May 11;12(3):e00366-21. doi: 10.1128/mBio.00366-21. mBio. 2021. PMID: 33975932 Free PMC article.
-
Effect of TLR agonist on infections bronchitis virus replication and cytokine expression in embryonated chicken eggs.Mol Immunol. 2020 Apr;120:52-60. doi: 10.1016/j.molimm.2020.02.001. Epub 2020 Feb 14. Mol Immunol. 2020. PMID: 32065987 Free PMC article.
-
Avian infectious bronchitis virus.Rev Sci Tech. 2000 Aug;19(2):493-508. doi: 10.20506/rst.19.2.1228. Rev Sci Tech. 2000. PMID: 10935276 Review.
-
Avian Cell Culture Models to Study Immunomodulatory Properties of Bioactive Products.Animals (Basel). 2022 Mar 7;12(5):670. doi: 10.3390/ani12050670. Animals (Basel). 2022. PMID: 35268238 Free PMC article. Review.
Cited by
-
Infectious bronchitis virus vaccination, but not the presence of XCR1, is correlated with large differences in chicken caecal microbiota.Microb Genom. 2024 Sep;10(9):001289. doi: 10.1099/mgen.0.001289. Microb Genom. 2024. PMID: 39222347 Free PMC article.
-
Fabrication of levofloxacin-loaded porcine acellular dermal matrix hydrogel and functional assessment in urinary tract infection.J Nanobiotechnology. 2024 Feb 7;22(1):52. doi: 10.1186/s12951-024-02322-w. J Nanobiotechnology. 2024. PMID: 38321555 Free PMC article.
-
Multiomics analysis reveals that microbiota regulate fat and muscle synthesis in chickens.Poult Sci. 2024 Mar;103(3):103417. doi: 10.1016/j.psj.2023.103417. Epub 2023 Dec 30. Poult Sci. 2024. PMID: 38218114 Free PMC article.
-
The dynamics of microbiome and virome in migratory birds of southwest China.NPJ Biofilms Microbiomes. 2025 Apr 23;11(1):64. doi: 10.1038/s41522-025-00703-z. NPJ Biofilms Microbiomes. 2025. PMID: 40268958 Free PMC article.
-
Pioneer colonizers: Bacteria that alter the chicken intestinal morphology and development of the microbiota.Front Physiol. 2023 Mar 29;14:1139321. doi: 10.3389/fphys.2023.1139321. eCollection 2023. Front Physiol. 2023. PMID: 37064908 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

