Mitochondria-Targeted Antioxidant Mitoquinone Maintains Mitochondrial Homeostasis through the Sirt3-Dependent Pathway to Mitigate Oxidative Damage Caused by Renal Ischemia/Reperfusion
- PMID: 36193071
- PMCID: PMC9526615
- DOI: 10.1155/2022/2213503
Mitochondria-Targeted Antioxidant Mitoquinone Maintains Mitochondrial Homeostasis through the Sirt3-Dependent Pathway to Mitigate Oxidative Damage Caused by Renal Ischemia/Reperfusion
Abstract
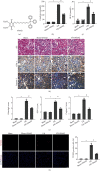
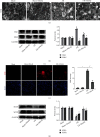
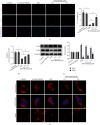
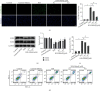
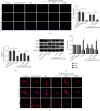
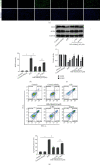
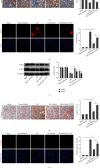
Mitochondrial dysfunction is a critical factor contributing to oxidative stress and apoptosis in ischemia-reperfusion (I/R) diseases. Mitoquinone (MitoQ) is a mitochondria-targeted antioxidant whose potent anti-I/R injury capacity has been demonstrated in organs such as the heart and the intestine. In the present study, we explored the role of MitoQ in maintaining mitochondrial homeostasis and attenuating oxidative damage in renal I/R injury. We discovered that the decreased renal function and pathological damage caused by renal I/R injury were significantly ameliorated by MitoQ. MitoQ markedly reversed mitochondrial damage after I/R injury and inhibited renal reactive oxygen species production. In vitro, hypoxia/reoxygenation resulted in increased mitochondrial fission and decreased mitochondrial fusion in human renal tubular epithelial cells (HK-2), which were partially prevented by MitoQ. MitoQ treatment inhibited oxidative stress and reduced apoptosis in HK-2 cells by restoring mitochondrial membrane potential, promoting ATP production, and facilitating mitochondrial fusion. Deeply, renal I/R injury led to a decreased expression of sirtuin-3 (Sirt3), which was recovered by MitoQ. Moreover, the inhibition of Sirt3 partially eliminated the protective effect of MitoQ on mitochondria and increased oxidative damage. Overall, our data demonstrate a mitochondrial protective effect of MitoQ, which raises the possibility of MitoQ as a novel therapy for renal I/R.
Copyright © 2022 Hu Mao et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

