Identification of Interleukin-1-Beta Inhibitors in Gouty Arthritis Using an Integrated Approach Based on Network Pharmacology, Molecular Docking, and Cell Experiments
- PMID: 36193152
- PMCID: PMC9526673
- DOI: 10.1155/2022/2322417
Identification of Interleukin-1-Beta Inhibitors in Gouty Arthritis Using an Integrated Approach Based on Network Pharmacology, Molecular Docking, and Cell Experiments
Abstract
Background: This study aimed to investigate the molecular mechanism of Tongfengding capsule (TFDC) in treating immune-inflammatory diseases of gouty arthritis (GA) and interleukin-1-beta (IL-1β) inhibitors by using network pharmacology, molecular docking, and cell experiments.
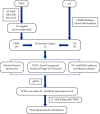
Methods: In this study, the compounds of TFDC and the potential inflammatory targets of GA were obtained from Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP), Online Mendelian Inheritance in Man (OMIM), and GeneCards databases. The TFDC-GA-potential targets interaction network was accomplished by the STRING database. The TFDC-active compound-potential target-GA network was constructed using Cytoscape software. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were used to further explore the GA mechanism and therapeutic effects of TFDC. Quantitative real-time PCR (qPCR) was used to verify whether the TFDC inhibited IL-1β in GA. Molecular docking technology was used to analyze the optimal effective compounds from the TFDC for docking with IL-1β.
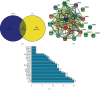
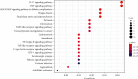
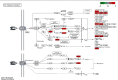
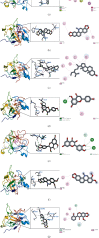
Result: 133 active compounds and 242 targets were screened from the TFDC, and 25 of the targets intersected with GA inflammatory targets, which were considered as potential therapeutic targets. Network pharmacological analysis showed that the TFDC active compounds such as quercetin, stigmasterol, betavulgarin, rutaecarpine, naringenin, dihydrochelerythrine, and dihydrosanguinarine had better correlation with GA inflammatory targets such as PTGS2, PTGS1, NOS2, SLC6A3, HTR3A, PPARG, MAPK14, RELA, MMP9, and MMP2. The immune-inflammatory signaling pathways of the active compounds for treating GA are IL-17 signaling pathway, TNF signaling pathway, NOD-like receptor signaling pathway, NF-kappa B signaling pathway, Toll-like receptor signaling pathway, HIF-1 signaling pathway, etc. The TFDC reduced IL-1β mRNA expression in GA by qPCR. Molecular docking results suggested that rutaecarpine was the most appropriate natural IL-1β inhibitor.
Conclusion: Our findings provide an essential role and bases for further immune-inflammatory studies on the molecular mechanisms of TFDC and IL-1β inhibitors development in GA.
Copyright © 2022 Liying Zeng et al.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures



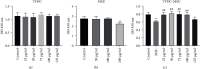

Similar articles
-
Exploration of the mechanism of Zisheng Shenqi decoction against gout arthritis using network pharmacology.Comput Biol Chem. 2021 Feb;90:107358. doi: 10.1016/j.compbiolchem.2020.107358. Epub 2020 Aug 8. Comput Biol Chem. 2021. PMID: 33243703 Review.
-
Network pharmacology and molecular docking to explore the treatment potential and molecular mechanism of Si-Miao decoction against gouty arthritis.Medicine (Baltimore). 2024 May 31;103(22):e38221. doi: 10.1097/MD.0000000000038221. Medicine (Baltimore). 2024. PMID: 39259129 Free PMC article.
-
Mechanism of ShuiJingDan in Treating Acute Gouty Arthritis Flares Based on Network Pharmacology and Molecular Docking.Drug Des Devel Ther. 2023 Nov 24;17:3493-3505. doi: 10.2147/DDDT.S436360. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 38034481 Free PMC article.
-
Identification of Tumor Necrosis Factor-Alpha (TNF-α) Inhibitor in Rheumatoid Arthritis Using Network Pharmacology and Molecular Docking.Front Pharmacol. 2021 May 21;12:690118. doi: 10.3389/fphar.2021.690118. eCollection 2021. Front Pharmacol. 2021. PMID: 34093213 Free PMC article.
-
Exploring the inhibition mechanism of interleukin-1-beta in gouty arthritis by polygonum cuspidatum using network pharmacology and molecular docking: A review.Medicine (Baltimore). 2023 Jul 21;102(29):e34396. doi: 10.1097/MD.0000000000034396. Medicine (Baltimore). 2023. PMID: 37478249 Free PMC article. Review.
Cited by
-
Elevated serum IL-2 and Th17/Treg imbalance are associated with gout.Clin Exp Med. 2024 Jan 19;24(1):9. doi: 10.1007/s10238-023-01253-4. Clin Exp Med. 2024. PMID: 38240927 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

