Application of physiologically-based pharmacokinetic model approach to predict pharmacokinetics and drug-drug interaction of rivaroxaban: A case study of rivaroxaban and carbamazepine
- PMID: 36193622
- PMCID: PMC9662201
- DOI: 10.1002/psp4.12844
Application of physiologically-based pharmacokinetic model approach to predict pharmacokinetics and drug-drug interaction of rivaroxaban: A case study of rivaroxaban and carbamazepine
Abstract
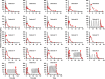
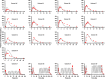
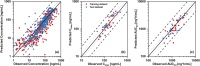
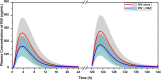
Rivaroxaban (RIV; Xarelto; Janssen Pharmaceuticals, Beerse, Belgium) is one of the direct oral anticoagulants. The drug is a strong substrate of cytochrome P450 (CYP) enzymes and efflux transporters. This study aimed to develop a physiologically-based pharmacokinetic (PBPK) model for RIV. It contained three hepatic metabolizing enzyme reactions (CYP3A4, CYP2J2, and CYP-independent) and two active transporter-mediated transfers (P-gp and BCRP transporters). To illustrate the performance of the developed RIV PBPK model on the prediction of drug-drug interactions (DDIs), carbamazepine (CBZ) was selected as a case study due to the high DDI potential. Our study results showed that CBZ significantly reduces the exposure of RIV. The area under the concentration-time curve from zero to infinity (AUCinf ) of RIV was reduced by 35.2% (from 2221.3 to 1438.7 ng*h/ml) and by 25.5% (from 2467.3 to 1838.4 ng*h/ml) after the first dose and at the steady-state, respectively, whereas the maximum plasma concentration (Cmax ) of RIV was reduced by 37.7% (from 266.3 to 166.1 ng/ml) and 36.4% (from 282.3 to 179.5 ng/ml), respectively. The developed PBPK model of RIV could be paired with PBPK models of other interested perpetrators to predict DDI profiles. Further studies investigating the extent of DDI between CBZ and RIV should be conducted in humans to gain a full understanding of their safety and effects.
© 2022 The Authors. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests in this work.
Figures

References
-
- Bayer Pharma AG Xarelto (rivaroxaban) Summary of Product Characteristics ; 2013. https://www.ema.europa.eu/en/documents/product‐information/xarelto‐epar‐...
-
- European Medicines Agency . CHMP assessment report for Xarelto ; 2008. https://www.ema.europa.eu/en/documents/assessment‐report/xarelto‐epar‐pu...
-
- Gnoth MJ, Buetehorn U, Muenster U, Schwarz T, Sandmann S. In vitro and in vivo P‐glycoprotein transport characteristics of rivaroxaban. J Pharmacol Exp Ther. 2011;338:372‐380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

