Kinetic Characterization and Inhibition of Trypanosoma cruzi Hypoxanthine-Guanine Phosphoribosyltransferases
- PMID: 36193631
- PMCID: PMC9536471
- DOI: 10.1021/acs.biochem.2c00312
Kinetic Characterization and Inhibition of Trypanosoma cruzi Hypoxanthine-Guanine Phosphoribosyltransferases
Abstract
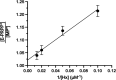
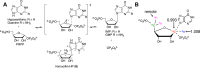
Chagas disease, caused by the parasitic protozoan Trypanosoma cruzi, affects over 8 million people worldwide. Current antiparasitic treatments for Chagas disease are ineffective in treating advanced, chronic stages of the disease, and are noted for their toxicity. Like most parasitic protozoa, T. cruzi is unable to synthesize purines de novo, and relies on the salvage of preformed purines from the host. Hypoxanthine-guanine phosphoribosyltransferases (HGPRTs) are enzymes that are critical for the salvage of preformed purines, catalyzing the formation of inosine monophosphate (IMP) and guanosine monophosphate (GMP) from the nucleobases hypoxanthine and guanine, respectively. Due to the central role of HGPRTs in purine salvage, these enzymes are promising targets for the development of new treatment methods for Chagas disease. In this study, we characterized two gene products in the T. cruzi CL Brener strain that encodes enzymes with functionally identical HGPRT activities in vitro: TcA (TcCLB.509693.70) and TcC (TcCLB.506457.30). The TcC isozyme was kinetically characterized to reveal mechanistic details on catalysis, including identification of the rate-limiting step(s) of catalysis. Furthermore, we identified and characterized inhibitors of T. cruzi HGPRTs originally developed as transition-state analogue inhibitors (TSAIs) of Plasmodium falciparum hypoxanthine-guanine-xanthine phosphoribosyltransferase (PfHGXPRT), where the most potent compound bound to T. cruzi HGPRT with low nanomolar affinity. Our results validated the repurposing of TSAIs to serve as selective inhibitors for orthologous molecular targets, where primary and secondary structures as well as putatively common chemical mechanisms are conserved.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Kinetic and Structural Characterization of Trypanosoma cruzi Hypoxanthine-Guanine-Xanthine Phosphoribosyltransferases and Repurposing of Transition-State Analogue Inhibitors.Biochemistry. 2023 Jul 18;62(14):2182-2201. doi: 10.1021/acs.biochem.3c00116. Epub 2023 Jul 7. Biochemistry. 2023. PMID: 37418678 Free PMC article.
-
Molecular and biochemical studies on the hypoxanthine-guanine phosphoribosyltransferases of the pathogenic haemoflagellates.Int J Parasitol. 1997 Feb;27(2):203-13. doi: 10.1016/s0020-7519(96)00150-6. Int J Parasitol. 1997. PMID: 9088991
-
Molecular characterization and overexpression of the hypoxanthine-guanine phosphoribosyltransferase gene from Trypanosoma cruzi.Mol Biochem Parasitol. 1994 Jun;65(2):233-45. doi: 10.1016/0166-6851(94)90075-2. Mol Biochem Parasitol. 1994. PMID: 7969265
-
Hypoxanthine-guanine phosphoribosyltransferase as a therapeutic target in protozoal infections.Infect Agents Dis. 1995 Mar;4(1):29-40. Infect Agents Dis. 1995. PMID: 7728354 Review.
-
Chagas disease: progress and new perspectives.Curr Med Chem. 2010;17(5):423-52. doi: 10.2174/092986710790226101. Curr Med Chem. 2010. PMID: 20015038 Review.
Cited by
-
Inhibition and Mechanism of Plasmodium falciparum Hypoxanthine-Guanine-Xanthine Phosphoribosyltransferase.ACS Chem Biol. 2022 Dec 16;17(12):3407-3419. doi: 10.1021/acschembio.2c00546. Epub 2022 Nov 22. ACS Chem Biol. 2022. PMID: 36413975 Free PMC article.
-
Evaluation of purine-nucleoside degrading ability and in vivo uric acid lowering of Streptococcus thermophilus IDCC 2201, a novel antiuricemia strain.PLoS One. 2024 Feb 22;19(2):e0293378. doi: 10.1371/journal.pone.0293378. eCollection 2024. PLoS One. 2024. PMID: 38386624 Free PMC article.
-
Kinetic and Structural Characterization of Trypanosoma cruzi Hypoxanthine-Guanine-Xanthine Phosphoribosyltransferases and Repurposing of Transition-State Analogue Inhibitors.Biochemistry. 2023 Jul 18;62(14):2182-2201. doi: 10.1021/acs.biochem.3c00116. Epub 2023 Jul 7. Biochemistry. 2023. PMID: 37418678 Free PMC article.
-
TrypPROTACs Unlocking New Therapeutic Strategies for Chagas Disease.Pharmaceuticals (Basel). 2025 Jun 19;18(6):919. doi: 10.3390/ph18060919. Pharmaceuticals (Basel). 2025. PMID: 40573314 Free PMC article. Review.
-
Discovery of Novel Inhibitors of Cruzain Cysteine Protease of Trypanosoma cruzi.Curr Med Chem. 2024;31(16):2285-2308. doi: 10.2174/0109298673254864230921090519. Curr Med Chem. 2024. PMID: 37888814
References
-
- Center for Disease Control. American Trypanosomiasis (also known as Chagas Disease). https://www.cdc.gov/parasites/chagas/gen_info/detailed.html#intro (accessed April 17, 2022).
-
- Pan American Health Organization. Chagas Disease. https://www.paho.org/en/topics/chagas-disease (accessed March 28, 2022).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

