Fabrication and Functionalisation of Nanocarbon-Based Field-Effect Transistor Biosensors
- PMID: 36193790
- PMCID: PMC10092808
- DOI: 10.1002/cbic.202200282
Fabrication and Functionalisation of Nanocarbon-Based Field-Effect Transistor Biosensors
Abstract
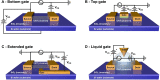
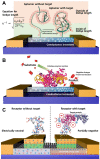
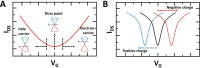
Nanocarbon-based field-effect transistor (NC-FET) biosensors are at the forefront of future diagnostic technology. By integrating biological molecules with electrically conducting carbon-based platforms, high sensitivity real-time multiplexed sensing is possible. Combined with their small footprint, portability, ease of use, and label-free sensing mechanisms, NC-FETs are prime candidates for the rapidly expanding areas of point-of-care testing, environmental monitoring and biosensing as a whole. In this review we provide an overview of the basic operational mechanisms behind NC-FETs, synthesis and fabrication of FET devices, and developments in functionalisation strategies for biosensing applications.
Keywords: biosensors; carbon nanotubes; field-effect transistors; graphene; point-of-care diagnostics; surface functionalisation.
© 2022 The Authors. ChemBioChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Advanced Materials for Biological Field-Effect Transistors (Bio-FETs) in Precision Healthcare and Biosensing.Adv Healthc Mater. 2025 May;14(13):e2500400. doi: 10.1002/adhm.202500400. Epub 2025 Apr 10. Adv Healthc Mater. 2025. PMID: 40207741 Free PMC article. Review.
-
2D Materials in Advanced Electronic Biosensors for Point-of-Care Devices.Adv Sci (Weinh). 2024 Aug;11(31):e2401386. doi: 10.1002/advs.202401386. Epub 2024 Jun 18. Adv Sci (Weinh). 2024. PMID: 38894575 Free PMC article. Review.
-
Toward the Commercialization of Carbon Nanotube Field Effect Transistor Biosensors.Biosensors (Basel). 2023 Feb 27;13(3):326. doi: 10.3390/bios13030326. Biosensors (Basel). 2023. PMID: 36979538 Free PMC article. Review.
-
Advances in field-effect biosensors towards point-of-use.Nanotechnology. 2023 Sep 25;34(49):492002. doi: 10.1088/1361-6528/acf3f0. Nanotechnology. 2023. PMID: 37625391 Free PMC article. Review.
-
A review on nanomaterial-based field effect transistor technology for biomarker detection.Mikrochim Acta. 2019 Nov 1;186(11):739. doi: 10.1007/s00604-019-3850-6. Mikrochim Acta. 2019. PMID: 31677098 Review.
Cited by
-
Two-Dimensional (2D) materials in the detection of SARS-CoV-2.Microchem J. 2023 Oct;193:108970. doi: 10.1016/j.microc.2023.108970. Epub 2023 Jun 14. Microchem J. 2023. PMID: 37342763 Free PMC article.
-
Advanced Materials for Biological Field-Effect Transistors (Bio-FETs) in Precision Healthcare and Biosensing.Adv Healthc Mater. 2025 May;14(13):e2500400. doi: 10.1002/adhm.202500400. Epub 2025 Apr 10. Adv Healthc Mater. 2025. PMID: 40207741 Free PMC article. Review.
-
An Updated Review on Electrochemical Nanobiosensors for Neurotransmitter Detection.Biosensors (Basel). 2023 Sep 19;13(9):892. doi: 10.3390/bios13090892. Biosensors (Basel). 2023. PMID: 37754127 Free PMC article. Review.
-
Click-Functionalization of Silanized Carbon Nanotubes: From Inorganic Heterostructures to Biosensing Nanohybrids.Molecules. 2023 Feb 25;28(5):2161. doi: 10.3390/molecules28052161. Molecules. 2023. PMID: 36903408 Free PMC article.
-
Modern Emerging Biosensing Methodologies for the Early Diagnosis and Screening of Ovarian Cancer.Biosensors (Basel). 2025 Mar 21;15(4):203. doi: 10.3390/bios15040203. Biosensors (Basel). 2025. PMID: 40277517 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
- BB/M009122/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/H003746/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- AC1910IF14/WT_/Wellcome Trust/United Kingdom
- BB/M000249/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources

