Multivalent glycocyclopeptides: conjugation methods and biological applications
- PMID: 36193815
- PMCID: PMC9575389
- DOI: 10.1039/d2cs00640e
Multivalent glycocyclopeptides: conjugation methods and biological applications
Abstract
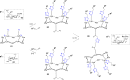
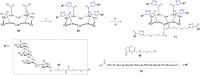
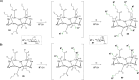
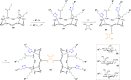
Click chemistry was extensively used to decorate synthetic multivalent scaffolds with glycans to mimic the cell surface glycocalyx and to develop applications in glycosciences. Conjugation methods such as oxime ligation, copper(I)-catalyzed alkyne-azide cycloaddition, thiol-ene coupling, squaramide coupling or Lansbury aspartylation proved particularly suitable to achieve this purpose. This review summarizes the synthetic strategies that can be used either in a stepwise manner or in an orthogonal one-pot approach, to conjugate multiple copies of identical or different glycans to cyclopeptide scaffolds (namely multivalent glycocyclopeptides) having different size, valency, geometry and molecular composition. The second part of this review will describe the potential of these structures to interact with various carbohydrate binding proteins or to stimulate immunity against tumor cells.
Conflict of interest statement
There are no conflicts to declare.
Figures

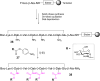


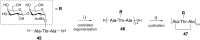
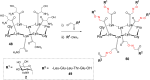
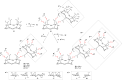



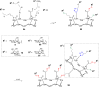
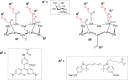
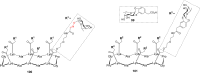
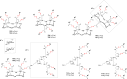
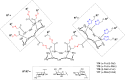
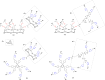
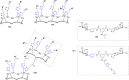

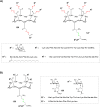
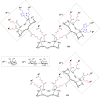
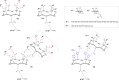
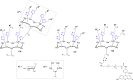
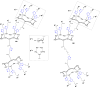




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

