A Qualitative Scoping Review of Early-Terminated Clinical Trials Sponsored by the Department of Veterans Affairs Cooperative Studies Program From 2010 to 2020
- PMID: 36193844
- PMCID: PMC10362930
- DOI: 10.1093/epirev/mxac009
A Qualitative Scoping Review of Early-Terminated Clinical Trials Sponsored by the Department of Veterans Affairs Cooperative Studies Program From 2010 to 2020
Abstract
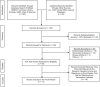
Increasing attention has been paid to the risks and benefits of terminating large clinical trials before reaching prespecified targets, because such decisions can greatly affect the implementation of findings. The Department of Veterans Affairs (VA) Cooperative Studies Program (CSP) is a research infrastructure dedicated to conducting high-quality clinical research. A scoping review was performed to characterize barriers preventing the attainment of prespecified recruitment, statistical power, or sample-size targets in VA CSP trials. A trial was eligible for inclusion if the trial was sponsored by the VA CSP, primary findings were published within the last 10 years, and a decision was made to terminate enrollment or follow-up before meeting a priori recruitment or endpoint targets. In 11 of 29 included trials (37.9%), a decision was made to terminate the trial early. The most common reason for early termination was related to under-recruitment (n = 5). Other reasons included early detection of safety signals (n = 2), futility (n = 1), and benefit (n = 1). This review highlights recruitment as a critical facet of trial conduct that may hinder the production of high-quality data and thus warrant additional attention. Solutions to enhance recruitment now implemented by the VA CSP, including dedicated enrollment infrastructure and screening facilitated by informatics approaches, show promise in reducing this cause for early termination.
Keywords: clinical trials; review; scoping review.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Johns Hopkins Bloomberg School of Public Health. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
An initiative using informatics to facilitate clinical research planning and recruitment in the VA health care system.Contemp Clin Trials Commun. 2018 Jul 10;11:107-112. doi: 10.1016/j.conctc.2018.07.001. eCollection 2018 Sep. Contemp Clin Trials Commun. 2018. PMID: 30035242 Free PMC article.
-
Department of Veterans Affairs Cooperative Studies Program Network of Dedicated Enrollment Sites: Implications for Surgical Trials.JAMA Surg. 2014 Jun;149(6):507-13. doi: 10.1001/jamasurg.2013.4150. JAMA Surg. 2014. PMID: 24647851
-
Development and implementation of standardized study performance metrics for a VA healthcare system clinical research consortium.Contemp Clin Trials. 2021 Sep;108:106505. doi: 10.1016/j.cct.2021.106505. Epub 2021 Jul 12. Contemp Clin Trials. 2021. PMID: 34265457
-
The role of the Veterans Affairs Medical Centers in patient care, surgical education, research and faculty development.Am J Surg. 2005 Nov;190(5):662-75. doi: 10.1016/j.amjsurg.2005.07.001. Am J Surg. 2005. PMID: 16226937 Review.
-
Comparing Quality of Surgical Care Between the US Department of Veterans Affairs and Non-Veterans Affairs Settings: A Systematic Review.J Am Coll Surg. 2023 Aug 1;237(2):352-361. doi: 10.1097/XCS.0000000000000720. Epub 2023 May 8. J Am Coll Surg. 2023. PMID: 37154441 Free PMC article.
Cited by
-
Collaborating to heal addiction and mental health in primary care (CHAMP): A protocol for a hybrid type 2a trial.Contemp Clin Trials. 2024 Nov;146:107700. doi: 10.1016/j.cct.2024.107700. Epub 2024 Sep 23. Contemp Clin Trials. 2024. PMID: 39322115
-
Reducing Sample Size While Improving Equity in Vaccine Clinical Trials: A Machine Learning-Based Recruitment Methodology with Application to Improving Trials of Hepatitis C Virus Vaccines in People Who Inject Drugs.Healthcare (Basel). 2024 Mar 13;12(6):644. doi: 10.3390/healthcare12060644. Healthcare (Basel). 2024. PMID: 38540608 Free PMC article.
References
-
- Huang GD, Ferguson RE, Peduzzi PN, et al. Scientific and organizational collaboration in comparative effectiveness research: the VA Cooperative Studies Program model. Am J Med. 2010;123(12 Suppl 1):e24–e31. - PubMed
-
- VA Cooperative Studies Program . VA Cooperative Studies Program (CSP). Published studies . April 2020. https://www.vacsp.research.va.gov/Published_Studies.asp. Accessed October 16, 2020.

