Dysregulation of integrin αvβ3 and α5β1 impedes migration of placental endothelial cells in fetal growth restriction
- PMID: 36193846
- PMCID: PMC9641665
- DOI: 10.1242/dev.200717
Dysregulation of integrin αvβ3 and α5β1 impedes migration of placental endothelial cells in fetal growth restriction
Abstract
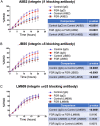
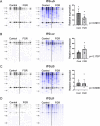
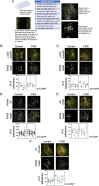
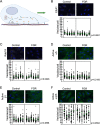
Placentas from pregnancies complicated by severe early-onset fetal growth restriction (FGR) exhibit diminished vascular development mediated by impaired angiogenesis, but underlying mechanisms remain unknown. In this study, we show that FGR endothelial cells demonstrate inherently reduced migratory capacity despite the presence of fibronectin, a matrix protein abundant in placental stroma that displays abnormal organization in FGR placentas. Thus, we hypothesized that aberrant endothelial-fibronectin interactions in FGR are a key mechanism underlying impaired FGR endothelial migration. Using human fetoplacental endothelial cells isolated from uncomplicated term control and FGR pregnancies, we assessed integrin α5β1 and αvβ3 regulation during cell migration. We show that endothelial integrin α5β1 and αvβ3 interactions with fibronectin are required for migration and that FGR endothelial cells responded differentially to integrin inhibition, indicating integrin dysregulation in FGR. Whole-cell expression was not different between groups. However, there were significantly more integrins in focal adhesions and reduced intracellular trafficking in FGR. These newly identified changes in FGR endothelial cellular processes represent previously unidentified mechanisms contributing to persistent angiogenic deficiencies in FGR.
Keywords: Angiogenesis; Endothelial cells; Fetal growth restriction; Human; Integrins; Placental dysfunction.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


Similar articles
-
Mechanistic insights into the development of severe fetal growth restriction.Clin Sci (Lond). 2023 Apr 26;137(8):679-695. doi: 10.1042/CS20220284. Clin Sci (Lond). 2023. PMID: 37186255 Free PMC article. Review.
-
Angiogenic Function of Human Placental Endothelial Cells in Severe Fetal Growth Restriction Is Not Rescued by Individual Extracellular Matrix Proteins.Cells. 2023 Sep 23;12(19):2339. doi: 10.3390/cells12192339. Cells. 2023. PMID: 37830553 Free PMC article.
-
Down-regulation of placental neuropilin-1 in fetal growth restriction.Am J Obstet Gynecol. 2016 Feb;214(2):279.e1-279.e9. doi: 10.1016/j.ajog.2015.09.068. Epub 2015 Sep 26. Am J Obstet Gynecol. 2016. PMID: 26409917
-
Constitutive Association of Tie1 and Tie2 with Endothelial Integrins is Functionally Modulated by Angiopoietin-1 and Fibronectin.PLoS One. 2016 Oct 3;11(10):e0163732. doi: 10.1371/journal.pone.0163732. eCollection 2016. PLoS One. 2016. PMID: 27695111 Free PMC article.
-
Angiogenic factors in placentas from pregnancies complicated by fetal growth restriction (review).Mol Med Rep. 2012 Jul;6(1):23-7. doi: 10.3892/mmr.2012.898. Epub 2012 May 2. Mol Med Rep. 2012. PMID: 22552373 Review.
Cited by
-
Mechanistic insights into the development of severe fetal growth restriction.Clin Sci (Lond). 2023 Apr 26;137(8):679-695. doi: 10.1042/CS20220284. Clin Sci (Lond). 2023. PMID: 37186255 Free PMC article. Review.
-
The potential role of the αVβ3 integrin receptor in placental biology and normal and complicated pregnancies.Br J Haematol. 2025 Apr;206(4):1054-1061. doi: 10.1111/bjh.20019. Epub 2025 Feb 20. Br J Haematol. 2025. PMID: 39976156 Review.
-
Integrins as a bridge between bacteria and cells: key targets for therapeutic wound healing.Burns Trauma. 2024 Jul 16;12:tkae022. doi: 10.1093/burnst/tkae022. eCollection 2024. Burns Trauma. 2024. PMID: 39015251 Free PMC article. Review.
-
Angiogenic Function of Human Placental Endothelial Cells in Severe Fetal Growth Restriction Is Not Rescued by Individual Extracellular Matrix Proteins.Cells. 2023 Sep 23;12(19):2339. doi: 10.3390/cells12192339. Cells. 2023. PMID: 37830553 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

