Maize Seedling Growth and Hormone Response Assays Using the Rolled Towel Method
- PMID: 36194012
- PMCID: PMC11648833
- DOI: 10.1002/cpz1.562
Maize Seedling Growth and Hormone Response Assays Using the Rolled Towel Method
Abstract
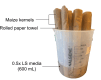
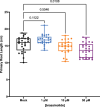
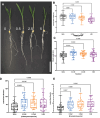
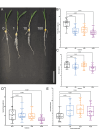
Root system architecture is a critical factor in maize health and stress resilience. Determining the genetic and environmental factors that shape maize root system architecture is an active research area. However, the ability to phenotype juvenile root systems is hindered by the use of field-grown and soil-based systems. An alternative to soil- and field-based growing conditions for maize seedlings is a controlled environment with a soil-free medium, which can facilitate root system phenotyping. Here, we describe how to grow maize under soil-free conditions for up to 12 days to facilitate root phenotyping. Maize seeds are sterilized and planted on specialized seed germination paper to minimize fungal contamination and ensure synchronized seedling growth, followed by imaging at the desired time point. The root images are then analyzed to quantify traits of interest, such as primary root length, lateral root density, seminal root length, and seminal root number. In addition, juvenile shoot traits can be quantified using manual annotation methods. We also outline the steps for performing rigorous hormone response assays for four classical phytohormones: auxin, brassinosteroid, cytokinin, and jasmonic acid. This protocol can be rapidly scaled up and is compatible with genetic screens and sample collection for downstream molecular analyses such as transcriptomics and proteomics. © 2022 The Authors. Current Protocols published by Wiley Periodicals LLC. Basic Protocol 1: Maize seedling rolled towel assay and phenotyping Basic Protocol 2: Maize seedling hormone response assays using the rolled towel assay.
Keywords: auxin; brassinosteroid; cytokinin; jasmonic acid; maize.
© 2022 The Authors. Current Protocols published by Wiley Periodicals LLC.
Conflict of interest statement
The authors have no financial or personal relationships or supporting sponsors to disclose.
Figures


References
Literature Cited
-
- Ahmad, R. M. , Cheng, C. , Sheng, J. , Wang, W. , Ren, H. , Aslam, M. , & Yan, Y. (2019). Interruption of jasmonic acid biosynthesis causes differential responses in the roots and shoots of maize seedlings against salt stress. International Journal of Molecular Sciences, 20, 6202. doi: 10.3390/ijms20246202 - DOI - PMC - PubMed
-
- von Behrens, I. , Komatsu, M. , Zhang, Y. , Berendzen, K. W. , Niu, X. , Sakai, H. , … Hochholdinger, F. (2011). Rootless with undetectable meristem 1 encodes a monocot‐specific AUX/IAA protein that controls embryonic seminal and post‐embryonic lateral root initiation in maize: AUX/IAA regulation of maize root development. The Plant Journal, 66, 341–353. doi: 10.1111/j.1365-313X.2011.04495.x - DOI - PubMed
Internet Resources
-
- ImageJ (also known as Fiji) Image Processing and Analysis in Java tool for measuring organ lengths.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

