Multicenter Reproducibility of Liver Iron Quantification with 1.5-T and 3.0-T MRI
- PMID: 36194113
- PMCID: PMC9885339
- DOI: 10.1148/radiol.213256
Multicenter Reproducibility of Liver Iron Quantification with 1.5-T and 3.0-T MRI
Abstract
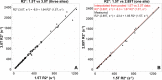
Background MRI is a standard of care tool to measure liver iron concentration (LIC). Compared with regulatory-approved R2 MRI, R2* MRI has superior speed and is available in most MRI scanners; however, the cross-vendor reproducibility of R2*-based LIC estimation remains unknown. Purpose To evaluate the reproducibility of LIC via single-breath-hold R2* MRI at both 1.5 T and 3.0 T with use of a multicenter, multivendor study. Materials and Methods Four academic medical centers using MRI scanners from three different vendors (three 1.5-T scanners, one 2.89-T scanner, and two 3.0-T scanners) participated in this prospective cross-sectional study. Participants with known or suspected liver iron overload were recruited to undergo multiecho gradient-echo MRI for R2* mapping at 1.5 T and 3.0 T (2.89 T or 3.0 T) on the same day. R2* maps were reconstructed from the multiecho images and analyzed at a single center. Reference LIC measurements were obtained with a commercial R2 MRI method performed using standardized 1.5-T spin-echo imaging. R2*-versus-LIC calibrations were generated across centers and field strengths using linear regression and compared using F tests. Receiver operating characteristic (ROC) curve analysis was used to determine the diagnostic performance of R2* MRI in the detection of clinically relevant LIC thresholds. Results A total of 207 participants (mean age, 38 years ± 20 [SD]; 117 male participants) were evaluated between March 2015 and September 2019. A linear relationship was confirmed between R2* and LIC. All calibrations within the same field strength were highly reproducible, showing no evidence of statistically significant center-specific differences (P > .43 across all comparisons). Calibrations for 1.5 T and 3.0 T were generated, as follows: for 1.5 T, LIC (in milligrams per gram [dry weight]) = -0.16 + 2.603 × 10-2 R2* (in seconds-1); for 2.89 T, LIC (in milligrams per gram) = -0.03 + 1.400 × 10-2 R2* (in seconds-1); for 3.0 T, LIC (in milligrams per gram) = -0.03 + 1.349 × 10-2 R2* (in seconds-1). Liver R2* had high diagnostic performance in the detection of clinically relevant LIC thresholds (area under the ROC curve, >0.98). Conclusion R2* MRI enabled accurate and reproducible quantification of liver iron overload over clinically relevant ranges of liver iron concentration (LIC). The data generated in this study provide the necessary calibrations for broad clinical dissemination of R2*-based LIC quantification. ClinicalTrials.gov registration no.: NCT02025543 © RSNA, 2022 Online supplemental material is available for this article.
Conflict of interest statement
Figures






References
-
- Angelucci E , Brittenham GM , McLaren CE , et al. . Hepatic iron concentration and total body iron stores in thalassemia major . N Engl J Med 2000. ; 343 ( 5 ): 327 – 331 . - PubMed
-
- Harmatz P , Butensky E , Quirolo K , et al. . Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy . Blood 2000. ; 96 ( 1 ): 76 – 79 . - PubMed
-
- Nielsen P , Günther U , Dürken M , Fischer R , Düllmann J . Serum ferritin iron in iron overload and liver damage: correlation to body iron stores and diagnostic relevance . J Lab Clin Med 2000. ; 135 ( 5 ): 413 – 418 . - PubMed
-
- Karam LB , Disco D , Jackson SM , et al. . Liver biopsy results in patients with sickle cell disease on chronic transfusions: poor correlation with ferritin levels . Pediatr Blood Cancer 2008. ; 50 ( 1 ): 62 – 65 . - PubMed
-
- Villeneuve JP , Bilodeau M , Lepage R , Côté J , Lefebvre M . Variability in hepatic iron concentration measurement from needle-biopsy specimens . J Hepatol 1996. ; 25 ( 2 ): 172 – 177 . - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

