Effect of a Postoperative Multimodal Opioid-Sparing Protocol vs Standard Opioid Prescribing on Postoperative Opioid Consumption After Knee or Shoulder Arthroscopy: A Randomized Clinical Trial
- PMID: 36194219
- PMCID: PMC9533185
- DOI: 10.1001/jama.2022.16844
Effect of a Postoperative Multimodal Opioid-Sparing Protocol vs Standard Opioid Prescribing on Postoperative Opioid Consumption After Knee or Shoulder Arthroscopy: A Randomized Clinical Trial
Erratum in
-
Incorrect Data.JAMA. 2022 Dec 13;328(22):2274. doi: 10.1001/jama.2022.21727. JAMA. 2022. PMID: 36511939 Free PMC article. No abstract available.
Abstract
Importance: In arthroscopic knee and shoulder surgery, there is growing evidence that opioid-sparing protocols may reduce postoperative opioid consumption while adequately addressing patients' pain. However, there are a lack of prospective, comparative trials evaluating their effectiveness.
Objective: To evaluate the effect of a multimodal, opioid-sparing approach to postoperative pain management compared with the current standard of care in patients undergoing arthroscopic shoulder or knee surgery.
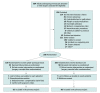
Design, setting, and participants: This randomized clinical trial was performed at 3 clinical sites in Ontario, Canada, and enrolled 200 patients from March 2021 to March 2022 with final follow-up completed in April 2022. Adult patients undergoing outpatient arthroscopic shoulder or knee surgery were followed up for 6 weeks postoperatively.
Interventions: The opioid-sparing group (100 participants randomized) received a prescription of naproxen, acetaminophen (paracetamol), and pantoprazole; a limited rescue prescription of hydromorphone; and a patient educational infographic. The control group (100 participants randomized) received the current standard of care determined by the treating surgeon, which consisted of an opioid analgesic.
Main outcomes and measures: The primary outcome was postoperative oral morphine equivalent (OME) consumption at 6 weeks after surgery. There were 5 secondary outcomes, including pain, patient satisfaction, opioid refills, quantity of OMEs prescribed at the time of hospital discharge, and adverse events at 6 weeks all reported at 6 weeks after surgery.
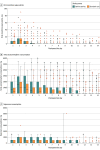
Results: Among the 200 patients who were randomized (mean age, 43 years; 73 women [38%]), 193 patients (97%) completed the trial; 98 of whom were randomized to receive standard care and 95 the opioid-sparing protocol. Patients in the opioid-sparing protocol consumed significantly fewer opioids (median, 0 mg; IQR, 0-8.0 mg) than patients in the control group (median, 40.0 mg; IQR, 7.5-105.0; z = -6.55; P < .001). Of the 5 prespecified secondary end points, 4 showed no significant difference. The mean amount of OMEs prescribed was 341.2 mg (95% CI, 310.2-372.2) in the standard care group and 40.4 mg (95% CI, 39.6-41.2) in the opioid-sparing group (mean difference, 300.8 mg; 95% CI, 269.4-332.3; P < .001). There was no significant difference in adverse events at 6 weeks (2 events [2.1%] in the standard care group vs 3 events [3.2%] in the opioid-sparing group), but more patients reported medication-related adverse effects in the standard care group (32% vs 19%, P = .048).
Conclusions and relevance: Among patients who underwent arthroscopic knee or shoulder surgery, a multimodal opioid-sparing postoperative pain management protocol, compared with standard opioid prescribing, significantly reduced postoperative opioid consumption over 6 weeks.
Trial registration: ClinicalTrials.gov Identifier: NCT04566250.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

