Prevalence of NTRK Fusions in Canadian Solid Tumour Cancer Patients
- PMID: 36194351
- PMCID: PMC9531629
- DOI: 10.1007/s40291-022-00617-y
Prevalence of NTRK Fusions in Canadian Solid Tumour Cancer Patients
Abstract
Introduction: Neurotrophic tyrosine receptor kinase (NTRK) gene fusions occur in ~ 0.3% of all solid tumours but are enriched in some rare tumour types. Tropomyosin receptor kinase (TRK) inhibitors larotrectinib and entrectinib are approved as tumour-agnostic therapies for solid tumours harbouring NTRK fusions.
Methods: This study investigated the prevalence of NTRK fusions in Canadian patients and also aimed to help guide NTRK testing paradigms through analysis of data reported from a national clinical diagnostic testing program between September 2019 and July 2021.
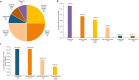
Results: Of 1,687 patients included in the final analysis, NTRK fusions were detected in 0.71% (n = 12) of patients representing salivary gland carcinoma (n = 3), soft tissue sarcoma (n = 3), CNS (n = 3), and one in each of melanoma, lung, and colorectal cancer. All three salivary gland carcinomas contained ETV6-NTRK3 fusions. Thirteen (0.77%) clinically actionable incidental findings were also detected. Two of the 13 samples containing incidental findings were NTRK fusion-positive (GFOD1-NTRK2, FGFR3-TACC3 in a glioblastoma and AFAP1-NTRK2, BRAF c.1799T>A in a glioma). The testing algorithm screened most patient samples via pan-TRK immunohistochemistry (IHC), whereas samples from the central nervous system (CNS), pathognomonic cancers, and confirmed/ putative NTRK fusion-positive samples identified under research protocols were reflexed straight to next-generation sequencing (NGS).
Conclusion: These findings highlight the benefit and practicality of a diagnostic testing program to identify patients suitable for tumour-agnostic TRK inhibitor therapies, as well as other targeted therapies, due to clinically actionable incidental findings identified. Collectively, these findings may inform future guidance on selecting the appropriate testing approach per tumour type and on optimal NTRK testing algorithms.
© 2022. The Author(s).
Conflict of interest statement
Harriet Feilotter received funding from Bayer Inc. to support implementation of the
Figures


References
-
- Barbacid M, Lamballe F, Pulido D, Klein R. The trk family of tyrosine protein kinase receptors. Biochim Biophys Acta. 1991;1072:115–127. - PubMed
-
- Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov. 2015;5:25–34. doi: 10.1158/2159-8290.CD-14-0765. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

