Effect of Topical Antibiotics on Duration of Acute Infective Conjunctivitis in Children: A Randomized Clinical Trial and a Systematic Review and Meta-analysis
- PMID: 36194412
- PMCID: PMC9533187
- DOI: 10.1001/jamanetworkopen.2022.34459
Effect of Topical Antibiotics on Duration of Acute Infective Conjunctivitis in Children: A Randomized Clinical Trial and a Systematic Review and Meta-analysis
Abstract
Importance: Although topical antibiotics are often prescribed for treating acute infective conjunctivitis in children, their efficacy is uncertain.
Objective: To assess the efficacy of topical antibiotic therapy for acute infective conjunctivitis.
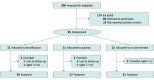
Design, setting, and participants: A randomized clinical trial was conducted in primary health care in Oulu, Finland, from October 15, 2014, to February 7, 2020. Children aged 6 months to 7 years with acute infective conjunctivitis were eligible for enrollment. The participants were followed up for 14 days. A subsequent meta-analysis included the present trial and 3 previous randomized clinical trials enrolling pediatric patients aged 1 month to 18 years with acute infective conjunctivitis.
Interventions: Participants in the present randomized clinical trial were randomized to moxifloxacin eye drops, placebo eye drops, or no intervention.
Main outcomes and measures: The primary outcome in the present randomized clinical trial was time to clinical cure (in days); in the meta-analysis, the primary outcome was the proportion of participants with conjunctival symptoms on days 3 to 6.
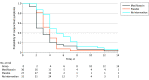
Results: The randomized clinical trial included 88 participants (46 [52%] girls), of whom 30 were randomized to moxifloxacin eye drops (mean [SD] age, 2.8 [1.6] years), 27 to placebo eye drops (mean [SD], age 3.0 [1.3] years), and 31 to no intervention (mean [SD] age, 3.2 [1.8] years). The time to clinical cure was significantly shorter in the moxifloxacin eye drop group than in the no intervention group (3.8 vs 5.7 days; difference, -1.9 days; 95% CI, -3.7 to -0.1 days; P = .04), while in the survival analysis both moxifloxacin and placebo eye drops significantly shortened the time to clinical cure relative to no intervention. In the meta-analysis, a total of 584 children were randomized (300 to topical antibiotics and 284 to a placebo), and the use of topical antibiotics was associated with a significant reduction in the proportion of children who had symptoms of conjunctivitis on days 3 to 6 compared with placebo eye drops (odds ratio, 0.59; 95% CI, 0.39 to 0.91).
Conclusions and relevance: In this randomized clinical trial and systematic review and meta-analysis, topical antibiotics were associated with significantly shorter durations of conjunctival symptoms in children with acute infective conjunctivitis.
Trial registration: ClinicalTrialsRegister.eu Identifier: 2013-005623-16.
Conflict of interest statement
Figures

Comment in
-
Topical antibiotics and artificial tears associated with reduced infective-conjunctivitis symptoms.J Pediatr. 2023 Oct;261:113320. doi: 10.1016/j.jpeds.2022.12.021. J Pediatr. 2023. PMID: 37741681 Free PMC article.
Similar articles
-
Efficacy and safety of besifloxacin ophthalmic suspension 0.6% in children and adolescents with bacterial conjunctivitis: a post hoc, subgroup analysis of three randomized, double-masked, parallel-group, multicenter clinical trials.Paediatr Drugs. 2010 Apr 1;12(2):105-12. doi: 10.2165/11534380-000000000-00000. Paediatr Drugs. 2010. PMID: 20218747
-
A randomised controlled trial of management strategies for acute infective conjunctivitis in general practice.BMJ. 2006 Aug 12;333(7563):321. doi: 10.1136/bmj.38891.551088.7C. Epub 2006 Jul 17. BMJ. 2006. PMID: 16847013 Free PMC article. Clinical Trial.
-
Chloramphenicol treatment for acute infective conjunctivitis in children in primary care: a randomised double-blind placebo-controlled trial.Lancet. 2005 Jul 2-8;366(9479):37-43. doi: 10.1016/S0140-6736(05)66709-8. Lancet. 2005. PMID: 15993231 Clinical Trial.
-
Interventions for bacterial folliculitis and boils (furuncles and carbuncles).Cochrane Database Syst Rev. 2021 Feb 26;2(2):CD013099. doi: 10.1002/14651858.CD013099.pub2. Cochrane Database Syst Rev. 2021. PMID: 33634465 Free PMC article.
-
Acute infective conjunctivitis in primary care: who needs antibiotics? An individual patient data meta-analysis.Br J Gen Pract. 2011 Sep;61(590):e542-8. doi: 10.3399/bjgp11X593811. Br J Gen Pract. 2011. PMID: 22152728 Free PMC article. Review.
Cited by
-
Topical Application onto the Eyelid Skin: Is it a Feasible Delivery Route of Ophthalmic Drugs?Mini Rev Med Chem. 2025;25(7):521-528. doi: 10.2174/0113895575358373241220043138. Mini Rev Med Chem. 2025. PMID: 39812048 Review.
-
The Effect of Ophthalmic Antibiotics on Clinical Outcomes and Transmissibility of Conjunctivitis Associated with Haemophilus influenzae versus Other Pathogens: Secondary Analysis of a Randomized Controlled Trial.J Pediatric Infect Dis Soc. 2024 Jul 20;13(7):349-351. doi: 10.1093/jpids/piae043. J Pediatric Infect Dis Soc. 2024. PMID: 38738667 Free PMC article. Clinical Trial.
-
Topical Antibiotic Therapy in the Ocular Environment: The Benefits of Using Moxifloxacin Eyedrops.Microorganisms. 2024 Mar 25;12(4):649. doi: 10.3390/microorganisms12040649. Microorganisms. 2024. PMID: 38674593 Free PMC article. Review.
-
Reducing Ophthalmic Antibiotic Use for Non-severe Conjunctivitis in Children.J Pediatric Infect Dis Soc. 2023 Sep 27;12(9):496-503. doi: 10.1093/jpids/piad065. J Pediatric Infect Dis Soc. 2023. PMID: 37696521 Free PMC article.
-
Pediatric Conjunctivitis: A Review of Clinical Manifestations, Diagnosis, and Management.Children (Basel). 2023 Apr 29;10(5):808. doi: 10.3390/children10050808. Children (Basel). 2023. PMID: 37238356 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

