Xenograft model of heterotopic transplantation of human ovarian cortical tissue and its clinical relevance
- PMID: 36194429
- PMCID: PMC9782463
- DOI: 10.1530/REP-22-0114
Xenograft model of heterotopic transplantation of human ovarian cortical tissue and its clinical relevance
Abstract
In brief: Xenografts of human ovarian cortical tissue provide a tractable model of heterotopic autotransplantation that is used for fertility preservation in patients undergoing ablative chemo/radiotherapy. This study describes the behavior of hundreds of xenografts to establish a framework for the clinical function of ovarian cortex following autotransplantation over short- and long-term intervals.
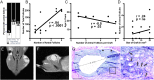
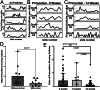
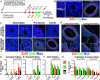
Abstract: More than 200 live births have been achieved using autotransplantation of cryopreserved ovarian cortical fragments, yet challenges remain to be addressed. Ischemia of grafted tissue undermines viability and longevity, typically requiring transplantation of multiple cortical pieces; and the dynamics of recruitment within a graft and the influence of parameters like size and patient age at the time of cryopreservation are not well-defined. Here, we describe results from a series of experiments in which we xenografted frozen/thawed human ovarian tissue (n = 440) from 28 girls and women (age range 32 weeks gestational age to 46 years, median 24.3 ± 4.6). Xenografts were recovered across a broad range of intervals (1-52 weeks post-transplantation) and examined histologically to quantify follicle density and distribution. The number of antral follicles in xenografted cortical fragments correlated positively with the total follicle number and was significantly reduced with increased patient age. Within xenografts, follicles were distributed in focal clusters, similar to the native ovary, but the presence of a leading antral follicle coincided with increased proliferation of surrounding follicles. These results underscore the importance of transplanting ovarian tissue with a high density of follicles and elucidate a potential paracrine influence of leading antral follicles on neighboring follicles of earlier stages. This temporal framework for interpreting the kinetics of follicle growth/mobilization may be useful in setting expectations and guiding the parameters of clinical autotransplantation.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures


Similar articles
-
Exogenous insulin-like growth factor 1 accelerates growth and maturation of follicles in human cortical xenografts and increases ovarian output in mice.F S Sci. 2021 Aug;2(3):237-247. doi: 10.1016/j.xfss.2021.07.002. Epub 2021 Jul 20. F S Sci. 2021. PMID: 35560275 Free PMC article.
-
Robot-assisted orthotopic and heterotopic ovarian tissue transplantation techniques: surgical advances since our first success in 2000.Fertil Steril. 2019 Mar;111(3):604-606. doi: 10.1016/j.fertnstert.2018.11.042. Fertil Steril. 2019. PMID: 30827527
-
N-acetylcysteine protects ovarian follicles from ischemia-reperfusion injury in xenotransplanted human ovarian tissue.Hum Reprod. 2021 Jan 25;36(2):429-443. doi: 10.1093/humrep/deaa291. Hum Reprod. 2021. PMID: 33246336
-
Cryopreservation and transplantation of ovarian tissue.Clin Obstet Gynecol. 2010 Dec;53(4):787-96. doi: 10.1097/GRF.0b013e3181f97a55. Clin Obstet Gynecol. 2010. PMID: 21048445 Review.
-
Orthotopic and heterotopic ovarian tissue transplantation.Best Pract Res Clin Obstet Gynaecol. 2010 Feb;24(1):113-26. doi: 10.1016/j.bpobgyn.2009.09.002. Epub 2009 Oct 23. Best Pract Res Clin Obstet Gynaecol. 2010. PMID: 19853515 Review.
Cited by
-
Classification system of human ovarian follicle morphology: recommendations of the National Institute of Child Health and Human Development - sponsored ovarian nomenclature workshop.Fertil Steril. 2025 May;123(5):761-778. doi: 10.1016/j.fertnstert.2024.11.016. Epub 2024 Nov 15. Fertil Steril. 2025. PMID: 39549739
-
Thawing fertility: a view of ovarian tissue cryopreservation processes and review of ovarian transplant research.Fertil Steril. 2024 Oct;122(4):574-585. doi: 10.1016/j.fertnstert.2024.07.005. Epub 2024 Jul 9. Fertil Steril. 2024. PMID: 38992745 Review.
-
Follicular development of fetal gonads under the skin of adult mice.Life Med. 2025 Feb 24;4(3):lnaf007. doi: 10.1093/lifemedi/lnaf007. eCollection 2025 Jun. Life Med. 2025. PMID: 40386496 Free PMC article.
References
-
- Bastings L, Beerendonk CC, Westphal JR, Massuger LF, Kaal SE, van Leeuwen FE, Braat DD, Peek R.2013Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: a systematic review. Human Reproduction Update 19483–506. (10.1093/humupd/dmt020) - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

