Increased soluble urokinase plasminogen activator levels modulate monocyte function to promote atherosclerosis
- PMID: 36194491
- PMCID: PMC9754000
- DOI: 10.1172/JCI158788
Increased soluble urokinase plasminogen activator levels modulate monocyte function to promote atherosclerosis
Abstract
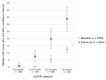
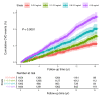
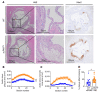
People with kidney disease are disproportionately affected by atherosclerosis for unclear reasons. Soluble urokinase plasminogen activator receptor (suPAR) is an immune-derived mediator of kidney disease, levels of which are strongly associated with cardiovascular outcomes. We assessed suPAR's pathogenic involvement in atherosclerosis using epidemiologic, genetic, and experimental approaches. We found serum suPAR levels to be predictive of coronary artery calcification and cardiovascular events in 5,406 participants without known coronary disease. In a genome-wide association meta-analysis including over 25,000 individuals, we identified a missense variant in the plasminogen activator, urokinase receptor (PLAUR) gene (rs4760), confirmed experimentally to lead to higher suPAR levels. Mendelian randomization analysis in the UK Biobank using rs4760 indicated a causal association between genetically predicted suPAR levels and atherosclerotic phenotypes. In an experimental model of atherosclerosis, proprotein convertase subtilisin/kexin-9 (Pcsk9) transfection in mice overexpressing suPAR (suPARTg) led to substantially increased atherosclerotic plaques with necrotic cores and macrophage infiltration compared with those in WT mice, despite similar cholesterol levels. Prior to induction of atherosclerosis, aortas of suPARTg mice excreted higher levels of CCL2 and had higher monocyte counts compared with WT aortas. Aortic and circulating suPARTg monocytes exhibited a proinflammatory profile and enhanced chemotaxis. These findings characterize suPAR as a pathogenic factor for atherosclerosis acting at least partially through modulation of monocyte function.
Keywords: Atherosclerosis; Cardiology; Innate immunity.
Figures



Comment in
-
Soluble urokinase plasminogen activator receptor (suPAR) promotes atherosclerosis.Kidney Int. 2023 Mar;103(3):451-454. doi: 10.1016/j.kint.2022.12.006. Epub 2022 Dec 19. Kidney Int. 2023. PMID: 36549362 No abstract available.
References
-
- Nykjaer A, et al. Urokinase receptor. An activation antigen in human T lymphocytes. J Immunol. 1994;152(2):505–516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI138347/AI/NIAID NIH HHS/United States
- R01 HL109946/HL/NHLBI NIH HHS/United States
- R35 HL155169/HL/NHLBI NIH HHS/United States
- 75N92020D00001/HL/NHLBI NIH HHS/United States
- N01 HC095167/HL/NHLBI NIH HHS/United States
- N02 HL64278/HL/NHLBI NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- R01 HL153384/HL/NHLBI NIH HHS/United States
- HHSN268201500003I/HL/NHLBI NIH HHS/United States
- 75N92020D00002/HL/NHLBI NIH HHS/United States
- HHSN268201500003C/HL/NHLBI NIH HHS/United States
- K99 AG068309/AG/NIA NIH HHS/United States
- N01HC95169/HL/NHLBI NIH HHS/United States
- N01 HC095161/HL/NHLBI NIH HHS/United States
- 75N92020D00005/HL/NHLBI NIH HHS/United States
- N01 HC095168/HL/NHLBI NIH HHS/United States
- T32 HL007853/HL/NHLBI NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- N02 HL064278/HL/NHLBI NIH HHS/United States
- N01 HC095159/HL/NHLBI NIH HHS/United States
- 75N92020D00003/HL/NHLBI NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- 75N92020D00004/HL/NHLBI NIH HHS/United States
- N01 HC095163/HL/NHLBI NIH HHS/United States
- 75N92020D00007/HL/NHLBI NIH HHS/United States
- UL1 TR002240/TR/NCATS NIH HHS/United States
- T32 AG062403/AG/NIA NIH HHS/United States
- K08 HL161448/HL/NHLBI NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- N01 HC095166/HL/NHLBI NIH HHS/United States
- MC_PC_17228/MRC_/Medical Research Council/United Kingdom
- N01 HC095162/HL/NHLBI NIH HHS/United States
- 75N92020D00006/HL/NHLBI NIH HHS/United States
- R01 DK128012/DK/NIDDK NIH HHS/United States
- R00 AG068309/AG/NIA NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- R01 AG028082/AG/NIA NIH HHS/United States
- N01 HC095165/HL/NHLBI NIH HHS/United States
- N01 HC095164/HL/NHLBI NIH HHS/United States
- R01 HL141399/HL/NHLBI NIH HHS/United States
- N01 HC095160/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

