IRF4 expression by lung dendritic cells drives acute but not Trm cell-dependent memory Th2 responses
- PMID: 36194494
- PMCID: PMC9675458
- DOI: 10.1172/jci.insight.140384
IRF4 expression by lung dendritic cells drives acute but not Trm cell-dependent memory Th2 responses
Abstract
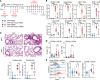
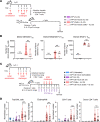
Expression of the transcription factor interferon regulatory factor 4 (IRF4) is required for the development of lung conventional DCs type 2 (cDC2s) that elicit Th2 responses, yet how IRF4 functions in lung cDC2s throughout the acute and memory allergic response is not clear. Here, we used a mouse model that loses IRF4 expression after lung cDC2 development to demonstrate that mice with IRF4-deficient DCs display impaired memory responses to allergen. This defect in the memory response was a direct result of ineffective Th2 induction and impaired recruitment of activated effector T cells to the lung after sensitization. IRF4-deficient DCs demonstrated defects in their migration to the draining lymph node and in T cell priming. Finally, T cells primed by IRF4-competent DCs mediated potent memory responses independently of IRF4-expressing DCs, demonstrating that IRF4-expressing DCs are not necessary during the memory response. Thus, IRF4 controlled a program in mature DCs governing Th2 priming and effector responses, but IRF4-expressing DCs were dispensable during tissue-resident memory T cell-dependent memory responses.
Keywords: Asthma; Dendritic cells; Immunology; Th2 response.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

