Enhanced immune responses following heterologous vaccination with self-amplifying RNA and mRNA COVID-19 vaccines
- PMID: 36194628
- PMCID: PMC9565686
- DOI: 10.1371/journal.ppat.1010885
Enhanced immune responses following heterologous vaccination with self-amplifying RNA and mRNA COVID-19 vaccines
Abstract
The optimal vaccination strategy to boost responses in the context of pre-existing immune memory to the SARS-CoV-2 spike (S) glycoprotein is an important question for global public health. To address this, we explored the SARS-CoV-2-specific humoral and cellular immune responses to a novel self-amplifying RNA (saRNA) vaccine followed by a UK authorised mRNA vaccine (BNT162b2) in individuals with and without previous COVID-19, and compared these responses with those who received an authorised vaccine alone. 35 subjects receiving saRNA (saRNA group) as part of the COVAC1 clinical trial and an additional 40 participants receiving an authorised SARS-CoV-2 vaccine only (non-saRNA group) were recruited. Antibody responses were measured by ELISA and a pseudoneutralisation assay for wildtype, Delta and Omicron variants. Cellular responses were measured by IFN-ƴ ELISpot and an activation induced marker (AIM) assay. Approximately 50% in each group had previous COVID-19 prior to vaccination, confirmed by PCR or antibody positivity on ELISA. All of those who received saRNA subsequently received a full course of an authorised vaccine. The majority (83%) of those receiving saRNA who were COVID-19 naïve at baseline seroconverted following the second dose, and those with previous COVID-19 had an increase in antibody titres two weeks following saRNA vaccination (median 27-fold), however titres were lower when compared to mRNA vaccination. Two weeks following the 2nd authorised mRNA vaccine dose, binding and neutralising antibody titres were significantly higher in the saRNA participants with previous COVID-19, compared to non-saRNA, or COVID-19 naive saRNA participants. Cellular responses were again highest in this group, with a higher proportion of spike specific CD8+ than CD4+ T cells when compared to those receiving the mRNA vaccine only. These findings suggest an immunological benefit of increased antigen exposure, both from natural infection and vaccination, particularly evident in those receiving heterologous vaccination with saRNA and mRNA.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
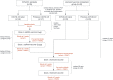
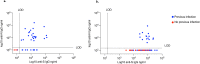


References
-
- Crotty S. Hybrid immunity. Science. 2021;372: 1392–1393. doi: 10.1126/SCIENCE.ABJ2258 - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

