Perivascular cells support folliculogenesis in the developing ovary
- PMID: 36194632
- PMCID: PMC9564831
- DOI: 10.1073/pnas.2213026119
Perivascular cells support folliculogenesis in the developing ovary
Abstract
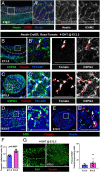
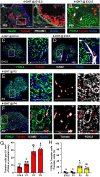
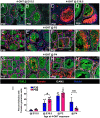
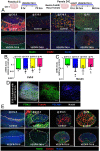
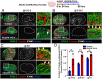
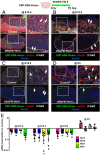
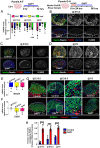
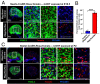
Supporting cells of the ovary, termed granulosa cells, are essential for ovarian differentiation and oogenesis by providing a nurturing environment for oocyte maintenance and maturation. Granulosa cells are specified in the fetal and perinatal ovary, and sufficient numbers of granulosa cells are critical for the establishment of follicles and the oocyte reserve. Identifying the cellular source from which granulosa cells and their progenitors are derived is an integral part of efforts to understand basic ovarian biology and the etiology of female infertility. In particular, the contribution of mesenchymal cells, especially perivascular cells, to ovarian development is poorly understood but is likely to be a source of new information regarding ovarian function. Here we have identified a cell population in the fetal ovary, which is a Nestin-expressing perivascular cell type. Using lineage tracing and ex vivo organ culture methods, we determined that perivascular cells are multipotent progenitors that contribute to granulosa, thecal, and pericyte cell lineages in the ovary. Maintenance of these progenitors is dependent on ovarian vasculature, likely reliant on endothelial-mesenchymal Notch signaling interactions. Depletion of Nestin+ progenitors resulted in a disruption of granulosa cell specification and in an increased number of germ cell cysts that fail to break down, leading to polyovular ovarian follicles. These findings highlight a cell population in the ovary and uncover a key role for vasculature in ovarian differentiation, which may lead to insights into the origins of female gonad dysgenesis and infertility.
Keywords: Nestin; blood vessels; ovarian follicle; ovary; perivascular cells.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
Role of Notch signaling in granulosa cell proliferation and polyovular follicle induction during folliculogenesis in mouse ovary.Cell Tissue Res. 2016 Jul;365(1):197-208. doi: 10.1007/s00441-016-2371-4. Epub 2016 Feb 22. Cell Tissue Res. 2016. PMID: 26899251
-
[Reconsidering the roles of female germ cells in ovarian development and folliculogenesis].Biol Aujourdhui. 2011;205(4):223-33. doi: 10.1051/jbio/2011022. Epub 2012 Jan 19. Biol Aujourdhui. 2011. PMID: 22251857 Review. French.
-
Directed differentiation of human iPSCs to functional ovarian granulosa-like cells via transcription factor overexpression.Elife. 2023 Feb 21;12:e83291. doi: 10.7554/eLife.83291. Elife. 2023. PMID: 36803359 Free PMC article.
-
Ovarian granulosa cell survival and proliferation requires the gonad-selective TFIID subunit TAF4b.Dev Biol. 2007 Mar 15;303(2):715-26. doi: 10.1016/j.ydbio.2006.12.011. Epub 2006 Dec 9. Dev Biol. 2007. PMID: 17207475 Free PMC article.
-
Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors.Hum Reprod Update. 2016 Dec;23(1):1-18. doi: 10.1093/humupd/dmw039. Epub 2016 Oct 26. Hum Reprod Update. 2016. PMID: 27797914 Free PMC article. Review.
Cited by
-
Single-cell sequencing reveals the reproductive variations between primiparous and multiparous Hu ewes.J Anim Sci Biotechnol. 2023 Nov 14;14(1):144. doi: 10.1186/s40104-023-00941-1. J Anim Sci Biotechnol. 2023. PMID: 37964337 Free PMC article.
-
Single-cell transcriptomic profiling of the neonatal oviduct and uterus reveals new insights into upper Müllerian duct regionalization.FASEB J. 2024 May 15;38(9):e23632. doi: 10.1096/fj.202400303R. FASEB J. 2024. PMID: 38686936 Free PMC article.
-
Partial male-to-female reprogramming of mouse fetal testis by Sertoli cell ablation.Development. 2023 Jul 15;150(14):dev201660. doi: 10.1242/dev.201660. Epub 2023 Jul 17. Development. 2023. PMID: 37376880 Free PMC article.
-
LRRC4 Deficiency Drives Premature Ovarian Insufficiency by Disrupting Metabolic Homeostasis in Granulosa Cells.Adv Sci (Weinh). 2025 Jun;12(23):e2417717. doi: 10.1002/advs.202417717. Epub 2025 May 2. Adv Sci (Weinh). 2025. PMID: 40317712 Free PMC article.
-
The assembly and activation of the PANoptosome promote porcine granulosa cell programmed cell death during follicular atresia.J Anim Sci Biotechnol. 2024 Nov 5;15(1):147. doi: 10.1186/s40104-024-01107-3. J Anim Sci Biotechnol. 2024. PMID: 39497227 Free PMC article.
References
-
- Wilhelm D., Palmer S., Koopman P., Sex determination and gonadal development in mammals. Physiol. Rev. 87, 1–28 (2007). - PubMed
-
- Stévant I., Nef S., Genetic control of gonadal sex determination and development. Trends Genet. 35, 346–358 (2019). - PubMed
-
- Smith P., Wilhelm D., Rodgers R. J., Development of mammalian ovary. J. Endocrinol. 221, R145–R161 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

