Camrelizumab in patients with advanced non-squamous non-small cell lung cancer: a cost-effective analysis in China
- PMID: 36194670
- PMCID: PMC9362787
- DOI: 10.1136/bmjopen-2022-061592
Camrelizumab in patients with advanced non-squamous non-small cell lung cancer: a cost-effective analysis in China
Abstract
Objective: Camrelizumab is a selective, humanised, high-affinity IgG4 kappa monoclonal antibody against programmed cell death 1 that shows effective antitumour activity with acceptable toxicity in multiple tumour types. The CameL trial demonstrated that camrelizumab plus chemotherapy (CC) significantly prolonged the median progression-free survival and median overall survival versus chemotherapy alone (CA) in patients with advanced non-squamous non-small cell lung cancer (NSCLC). Our study was conducted to investigate the cost-effectiveness of the two strategies in chemotherapy-naive patients with advanced non-squamous NSCLC.
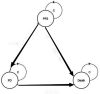
Design, setting and participants: A Markov simulation model was generated based on the CameL trial. The two simulated treatments included CC and CA.
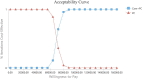
Primary and secondary outcome measures: Utility was derived from published literature, and costs were calculated based on those at our hospital in Chengdu, China. Incremental cost-effectiveness ratios (ICERs) were calculated to compare the cost-effectiveness of the two treatment arms.
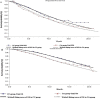
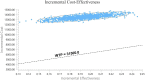
Results: In the overall population, the total costs were $27 223.40 and $13 740.10 for CC and CA treatment, respectively. The CC treatment produced 1.37 quality-adjusted life years (QALYs), and the CA treatment produced 1.17 QALYs. Hence, patients who were in the CC group spent an additional $13 483.30 and generated an increase of 0.20 QALYs, resulting in an ICER of $67 416.50 per QALY.
Conclusions: For chemotherapy-naive patients with advanced non-squamous NSCLC, CC is not considered a cost-effective treatment versus CA in China when considering a willingness-to-pay threshold of $31 500 per QALY.
Trial registration number: NCT03134872.
Keywords: CHEMOTHERAPY; HEALTH ECONOMICS; Health economics; Respiratory tract tumours.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

Similar articles
-
Economic Evaluation of First-Line Camrelizumab for Advanced Non-small-cell Lung Cancer in China.Front Public Health. 2021 Dec 10;9:743558. doi: 10.3389/fpubh.2021.743558. eCollection 2021. Front Public Health. 2021. PMID: 34957008 Free PMC article.
-
Cost-effectiveness analysis of pembrolizumab versus standard-of-care chemotherapy for first-line treatment of PD-L1 positive (>50%) metastatic squamous and non-squamous non-small cell lung cancer in France.Lung Cancer. 2019 Jan;127:44-52. doi: 10.1016/j.lungcan.2018.11.008. Epub 2018 Nov 23. Lung Cancer. 2019. PMID: 30642550
-
Cost-effectiveness analysis of camrelizumab plus chemotherapy as first-line treatment for advanced squamous NSCLC in China.Front Public Health. 2022 Aug 15;10:912921. doi: 10.3389/fpubh.2022.912921. eCollection 2022. Front Public Health. 2022. PMID: 36045725 Free PMC article.
-
Economics of first-line treatment with tislelizumab in patients with nonsquamous non-small cell lung cancer.Immunotherapy. 2024;16(20-22):1217-1226. doi: 10.1080/1750743X.2024.2433408. Epub 2024 Nov 28. Immunotherapy. 2024. PMID: 39606846 Free PMC article.
-
Pemetrexed for the maintenance treatment of locally advanced or metastatic non-small cell lung cancer.Health Technol Assess. 2010 Oct;14(Suppl. 2):33-9. doi: 10.3310/hta14suppl2/05. Health Technol Assess. 2010. PMID: 21047489 Review.
Cited by
-
Updated cost-effectiveness analysis of adebrelimab plus chemotherapy for extensive-stage small cell lung cancer in China.BMJ Open. 2024 Apr 5;14(4):e077090. doi: 10.1136/bmjopen-2023-077090. BMJ Open. 2024. PMID: 38582540 Free PMC article.
-
Anti-Programmed Cell Death-1 Versus Anti-Programmed Death-Ligand 1 (PD-L1) in PD-L1-Negative Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis.World J Oncol. 2025 Jun;16(3):299-310. doi: 10.14740/wjon2520. Epub 2025 Apr 22. World J Oncol. 2025. PMID: 40556968 Free PMC article.
-
Cost-effectiveness of camrelizumab plus chemotherapy vs. chemotherapy in the first-line treatment of non-squamous NSCLC: Evidence from China.Front Med (Lausanne). 2023 Feb 14;10:1122731. doi: 10.3389/fmed.2023.1122731. eCollection 2023. Front Med (Lausanne). 2023. PMID: 36865055 Free PMC article.
-
Hepatic cavernous hemangioma developed in non-small cell lung cancer patients after receiving Camrelizumab treatment: two case reports.Front Oncol. 2023 Aug 3;13:1221309. doi: 10.3389/fonc.2023.1221309. eCollection 2023. Front Oncol. 2023. PMID: 37601678 Free PMC article.
References
-
- Paz-Ares L, Brahmer J, Hellmann MD, et al. . CheckMate 227: a randomized, open-label phase 3 trial of nivolumab, nivolumab plus ipilimumab, or nivolumab plus chemotherapy versus chemotherapy in chemotherapy-naïve patients with advanced non-small cell lung cancer (NSCLC). Annals of Oncology 2017;28:ii50–1. 10.1093/annonc/mdx091.064 - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
