Advancing humanized monoclonal antibody for counteracting fentanyl toxicity towards clinical development
- PMID: 36194773
- PMCID: PMC9746415
- DOI: 10.1080/21645515.2022.2122507
Advancing humanized monoclonal antibody for counteracting fentanyl toxicity towards clinical development
Abstract
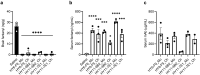
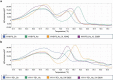
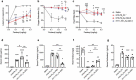
Innovative therapies to complement current treatments are needed to curb the growing incidence of fatal overdoses related to synthetic opioids. Murine and chimeric monoclonal antibodies (mAb) specific for fentanyl and its analogs have demonstrated pre-clinical efficacy in preventing and reversing drug-induced toxicity in rodent models. However, mAb-based therapeutics require extensive engineering as well as in vitro and in vivo characterization to advance to first-in-human clinical trials. Here, novel murine anti-fentanyl mAbs were selected for development based on affinity for fentanyl, and efficacy in counteracting the pharmacological effects of fentanyl in mice. Humanization and evaluation of mutations designed to eliminate predicted post-translational modifications resulted in two humanized mAbs that were effective at preventing fentanyl-induced pharmacological effects in rats. These humanized mAbs showed favorable biophysical properties with respect to aggregation and hydrophobicity by chromatography-based assays, and thermostability by dynamic scanning fluorimetry. These results collectively support that the humanized anti-fentanyl mAbs developed herein warrant further clinical development for treatment of fentanyl toxicity.
Keywords: PTM mitigation; antibody characterization; antibody engineering; clinical development; fentanyl; humanized antibody; mAb; manufacturability; opioid use disorder; synthetic opioids.
Conflict of interest statement
Baehr, Hicks, and Pravetoni are inventors of patent applications disclosing antibodies against fentanyl and its analogs, methods of making, and use thereof. Other authors declare no conflict of interest.
Figures


Similar articles
-
In vitro biophysical and pharmacological profiling predicts in vivo efficacy of anti-carfentanil monoclonal antibodies in mice.Biochem Biophys Res Commun. 2025 Jul 12;770:151995. doi: 10.1016/j.bbrc.2025.151995. Epub 2025 May 10. Biochem Biophys Res Commun. 2025. PMID: 40378616
-
Development of fentanyl-specific monoclonal antibody (mAb) to antagonize the pharmacological effects of fentanyl.Toxicol Appl Pharmacol. 2024 May;486:116918. doi: 10.1016/j.taap.2024.116918. Epub 2024 Apr 1. Toxicol Appl Pharmacol. 2024. PMID: 38570042
-
Monoclonal Antibodies for Combating Synthetic Opioid Intoxication.J Am Chem Soc. 2019 Jul 3;141(26):10489-10503. doi: 10.1021/jacs.9b04872. Epub 2019 Jun 25. J Am Chem Soc. 2019. PMID: 31187995 Free PMC article.
-
New anti-CD20 monoclonal antibodies for the treatment of B-cell lymphoid malignancies.BioDrugs. 2011 Feb 1;25(1):13-25. doi: 10.2165/11539590-000000000-00000. BioDrugs. 2011. PMID: 21090841 Review.
-
New monoclonal antibodies in renal transplantation.Minerva Urol Nefrol. 2003 Mar;55(1):57-66. Minerva Urol Nefrol. 2003. PMID: 12773967 Review.
Cited by
-
Investigation of monoclonal antibody CSX-1004 for fentanyl overdose.Nat Commun. 2023 Dec 5;14(1):7700. doi: 10.1038/s41467-023-43126-0. Nat Commun. 2023. PMID: 38052779 Free PMC article.
-
Catalytic Antibody Blunts Carfentanil-Induced Respiratory Depression.ACS Pharmacol Transl Sci. 2023 Apr 17;6(5):802-811. doi: 10.1021/acsptsci.3c00031. eCollection 2023 May 12. ACS Pharmacol Transl Sci. 2023. PMID: 37200811 Free PMC article.
-
Structure-Based Engineering of Monoclonal Antibodies for Improved Binding to Counteract the Effects of Fentanyl and Carfentanil.ACS Omega. 2024 Oct 7;9(41):42506-42519. doi: 10.1021/acsomega.4c06617. eCollection 2024 Oct 15. ACS Omega. 2024. PMID: 39431098 Free PMC article.
-
Substructure-Specific Antibodies Against Fentanyl Derivatives.ACS Nano. 2025 Jan 28;19(3):3714-3725. doi: 10.1021/acsnano.4c14369. Epub 2025 Jan 10. ACS Nano. 2025. PMID: 39792034 Free PMC article.
-
NK Cell and Monocyte Dysfunction in Multisystem Inflammatory Syndrome in Children.J Immunol. 2024 Nov 15;213(10):1452-1466. doi: 10.4049/jimmunol.2400395. J Immunol. 2024. PMID: 39392378 Free PMC article.
References
-
- Ahmad FB, Rossen LM, Sutton P.. Provisional drug overdose death counts; 2021.
-
- Centres for Disease Control and Prevention . Overdose death rates involving opioids, by type, United States, 1999-2019. National Center Injury Prev Control. 2021;2.
-
- O’Donnell J, Gladden RM, Goldberger BA, Mattson CL, Kariisa M. Notes from the field: opioid-involved overdose deaths with fentanyl or fentanyl analogs detected — 28 states and the District of Columbia, July 2016–december 2018. MMWR Morb Mortal Wkly Rep. 2020;69(10):1–15. doi:10.15585/mmwr.mm6910a4. - DOI - PMC - PubMed
-
- Shover CL, Falasinnu TO, Dwyer CL, Santos NB, Cunningham NJ, Freedman RB, Vest NA, Humphreys K. Steep increases in fentanyl-related mortality west of the Mississippi River: recent evidence from county and state surveillance. Drug Alcohol Depend. 2020;216:108314. doi:10.1016/j.drugalcdep.2020.108314. - DOI - PMC - PubMed
-
- Krotulski AJ, Chapman BP, Marks SJ, Ontiveros, ST, Devin-Holcombe, K, Fogarty, MF, Trieu, H, Logan, BK, Merchant, RC, Babu, KM. Sentanyl: a comparison of blood fentanyl concentrations and naloxone dosing after non-fatal overdose. Clin Toxicol. 2022;60(2):197–204. doi:10.1080/15563650.2021.1948558. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
