Validation of a non-oncogene encoded vulnerability to exportin 1 inhibition in pediatric renal tumors
- PMID: 36195086
- PMCID: PMC9669237
- DOI: 10.1016/j.medj.2022.09.002
Validation of a non-oncogene encoded vulnerability to exportin 1 inhibition in pediatric renal tumors
Abstract
Background: Malignant rhabdoid tumors (MRTs) and Wilms' tumors (WTs) are rare and aggressive renal tumors of infants and young children comprising ∼5% of all pediatric cancers. MRTs are among the most genomically stable cancers, and although WTs are genomically heterogeneous, both generally lack therapeutically targetable genetic mutations.
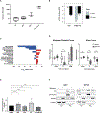
Methods: Comparative protein activity analysis of MRTs (n = 68) and WTs (n = 132) across TCGA and TARGET cohorts, using metaVIPER, revealed elevated exportin 1 (XPO1) inferred activity. In vitro studies were performed on a panel of MRT and WT cell lines to evaluate effects on proliferation and cell-cycle progression following treatment with the selective XPO1 inhibitor selinexor. In vivo anti-tumor activity was assessed in patient-derived xenograft (PDX) models of MRTs and WTs.
Findings: metaVIPER analysis identified markedly aberrant activation of XPO1 in MRTs and WTs compared with other tumor types. All MRT and most WT cell lines demonstrated baseline, aberrant XPO1 activity with in vitro sensitivity to selinexor via cell-cycle arrest and induction of apoptosis. In vivo, XPO1 inhibitors significantly abrogated tumor growth in PDX models, inducing effective disease control with sustained treatment. Corroborating human relevance, we present a case report of a child with multiply relapsed WTs with prolonged disease control on selinexor.
Conclusions: We report on a novel systems-biology-based comparative framework to identify non-genetically encoded vulnerabilities in genomically quiescent pediatric cancers. These results have provided preclinical rationale for investigation of XPO1 inhibitors in an upcoming investigator-initiated clinical trial of selinexor in children with MRTs and WTs and offer opportunities for exploration of inferred XPO1 activity as a potential predictive biomarker for response.
Funding: This work was funded by CureSearch for Children's Cancer, Alan B. Slifka Foundation, NIH (U01 CA217858, S10 OD012351, and S10 OD021764), Michael's Miracle Cure, Hyundai Hope on Wheels, Cannonball Kids Cancer, Conquer Cancer the ASCO Foundation, Cycle for Survival, Paulie Strong Foundation, and the Grayson Fund.
Keywords: PDX; Preclinical research; Wilms tumors; eltanexor; exportin 1; malignant rhabdoid tumors; patient-derived xenograft model; precision medicine; selinexor.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.C. is co-founder, equity holder, and consultant of DarwinHealth, Inc., which has licensed intellectual property (IP) related to the VIPER algorithms from Columbia University. Columbia University is an equity holder in DarwinHealth, Inc. A.L.K. is on the Scientific Advisory Board of Emendo Biotherapeutics, Karyopharm Therapeutics, Imago BioSciences, and DarwinHealth, is co-founder and on the board of directors of Isabl Technologies, and has equity interest in Imago BioSciences, Emendo Biotherapeutics, and Isabl Technologies.
Figures



Comment in
-
Exportin(g) precision oncology beyond genomics with XPO1 inhibition.Med. 2022 Nov 11;3(11):730-732. doi: 10.1016/j.medj.2022.10.005. Med. 2022. PMID: 36370692
References
-
- Grobner SN, Worst BC, Weischenfeldt J, et al.: The landscape of genomic alterations across childhood cancers. Nature 555:321–327, 2018 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

