Association between initiation of fluoroquinolones and hospital admission or emergency department visit for suicidality: population based cohort study
- PMID: 36195324
- PMCID: PMC9530980
- DOI: 10.1136/bmj-2021-069931
Association between initiation of fluoroquinolones and hospital admission or emergency department visit for suicidality: population based cohort study
Abstract
Objective: To evaluate the association between initiation of fluoroquinolones and hospital admission or emergency department visit for suicidality.
Design: Population based cohort study.
Setting: IBM MarketScan database, USA.
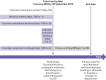
Participants: 2 756 268 adults (≥18 years) who initiated an oral fluoroquinolone (ciprofloxacin, levofloxacin, moxifloxacin, gemifloxacin, ofloxacin, gatifloxacin, norfloxacin, lomefloxacin, besifloxacin) or comparator antibiotic (January 2003 to September 2015) and had at least six months of continuous health plan enrollment and a diagnosis of pneumonia or urinary tract infection (UTI) three days or less before the drug initiation date. Comparator antibiotics were azithromycin in the pneumonia cohort and trimethoprim-sulfamethoxazole in the UTI cohort. Participants were matched 1:1 within each cohort on a propensity score, calculated from a multivariable logistic regression model that included 57 baseline covariates.
Main outcomes measure: Primary outcome was hospital admission or emergency department visit for suicidal ideation or self-harm within 60 days after treatment initiation. Cox proportional hazard models were used to estimate hazard ratios and 95% confidence intervals.
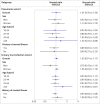
Results: The pneumonia cohort included 551 042 individuals, and the UTI cohort included 2 205 526 individuals. During the 60 day follow-up, 181 events were observed in the pneumonia cohort and 966 in the UTI cohort. The adjusted hazard ratios for fluoroquinolones were 1.01 (95% confidence interval 0.76 to 1.36) versus azithromycin in the pneumonia cohort and 1.03 (0.91 to 1.17) versus trimethoprim-sulfamethoxazole in the UTI cohort. Results were consistent across sensitivity analyses and subgroups of sex, age, or history of mental illnesses.
Conclusion: Initiation of fluoroquinolones was not associated with a substantially increased risk of admission to hospital or emergency department visits for suicidality compared with azithromycin or trimethoprim-sulfamethoxazole.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital for the submitted work. JJG previously received salary support from investigator initiated grants from Eli Lilly and Novartis Pharmaceutical awarded to the Brigham and Women’s Hospital for projects not related to the study. JJG was previously a consultant for Optum, was previously employed by Exponent, and is currently an employee of Johnson & Johnson and reports stock options (Johnson & Johnson), all unrelated to the study.
Figures

References
-
- Cowling T, Farrah K. Fluoroquinolones for the Treatment of Other Respiratory Tract Infections: A Review of Clinical Effectiveness. Cost-Effectiveness, and Guidelines, 2019.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
