Active Licking Shapes Cortical Taste Coding
- PMID: 36195439
- PMCID: PMC9671578
- DOI: 10.1523/JNEUROSCI.0942-22.2022
Active Licking Shapes Cortical Taste Coding
Abstract
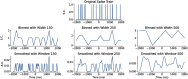
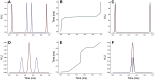
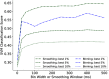
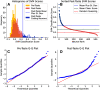
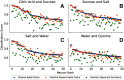
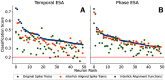
Neurons in the gustatory cortex (GC) represent taste through time-varying changes in their spiking activity. The predominant view is that the neural firing rate represents the sole unit of taste information. It is currently not known whether the phase of spikes relative to lick timing is used by GC neurons for taste encoding. To address this question, we recorded spiking activity from >500 single GC neurons in male and female mice permitted to freely lick to receive four liquid gustatory stimuli and water. We developed a set of data analysis tools to determine the ability of GC neurons to discriminate gustatory information and then to quantify the degree to which this information exists in the spike rate versus the spike timing or phase relative to licks. These tools include machine learning algorithms for classification of spike trains and methods from geometric shape and functional data analysis. Our results show that while GC neurons primarily encode taste information using a rate code, the timing of spikes is also an important factor in taste discrimination. A further finding is that taste discrimination using spike timing is improved when the timing of licks is considered in the analysis. That is, the interlick phase of spiking provides more information than the absolute spike timing itself. Overall, our analysis demonstrates that the ability of GC neurons to distinguish among tastes is best when spike rate and timing is interpreted relative to the timing of licks.SIGNIFICANCE STATEMENT Neurons represent information from the outside world via changes in their number of action potentials (spikes) over time. This study examines how neurons in the mouse gustatory cortex (GC) encode taste information when gustatory stimuli are experienced through the active process of licking. We use electrophysiological recordings and data analysis tools to evaluate the ability of GC neurons to distinguish tastants and then to quantify the degree to which this information exists in the spike rate versus the spike timing relative to licks. We show that the neuron's ability to distinguish between tastes is higher when spike rate and timing are interpreted relative to the timing of licks, indicating that the lick cycle is a key factor for taste processing.
Keywords: coding; elastic shape analysis; gustatory cortex; licking; support vector machine; taste.
Copyright © 2022 Neese et al.
Figures

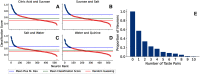
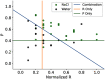
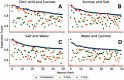
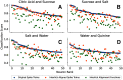

References
-
- Bauer M, Bruveris M, Michor PW (2016) Uniqueness of the Fisher–Rao metric on the space of smooth densities. Bull London Math Soc 48:499–506. 10.1112/blms/bdw020 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
