Gain-of-function mutations in KCNK3 cause a developmental disorder with sleep apnea
- PMID: 36195757
- PMCID: PMC9534757
- DOI: 10.1038/s41588-022-01185-x
Gain-of-function mutations in KCNK3 cause a developmental disorder with sleep apnea
Abstract
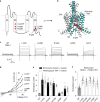
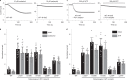
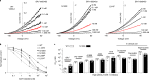
Sleep apnea is a common disorder that represents a global public health burden. KCNK3 encodes TASK-1, a K+ channel implicated in the control of breathing, but its link with sleep apnea remains poorly understood. Here we describe a new developmental disorder with associated sleep apnea (developmental delay with sleep apnea, or DDSA) caused by rare de novo gain-of-function mutations in KCNK3. The mutations cluster around the 'X-gate', a gating motif that controls channel opening, and produce overactive channels that no longer respond to inhibition by G-protein-coupled receptor pathways. However, despite their defective X-gating, these mutant channels can still be inhibited by a range of known TASK channel inhibitors. These results not only highlight an important new role for TASK-1 K+ channels and their link with sleep apnea but also identify possible therapeutic strategies.
© 2022. The Author(s).
Conflict of interest statement
M.G.H. and T.M. are employees of Bayer AG and are involved in the development of TASK-1 inhibitors for the treatment of sleep apnea. M.E.H. is scientific director of Congenica. A.B. and R.W. are employees of GeneDx, Inc. The other authors declare no conflicts of interest.
Figures


Comment in
-
Genomic insights into TASK-1 reveal functional roles in sleep apnea.Nat Genet. 2022 Oct;54(10):1451-1452. doi: 10.1038/s41588-022-01195-9. Nat Genet. 2022. PMID: 36195756 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

