EVI1 expression in early-stage breast cancer patients treated with neoadjuvant chemotherapy
- PMID: 36195836
- PMCID: PMC9533588
- DOI: 10.1186/s12885-022-10109-1
EVI1 expression in early-stage breast cancer patients treated with neoadjuvant chemotherapy
Abstract
Background: Overexpression of the EVI1 (ecotropic viral integration site 1) oncogene has recently been implicated as a prognostic factor in breast cancer (BC), particularly in triple-negative BC (TNBC). In this study we aimed to investigate frequency and clinical relevance of EVI1 expression in newly diagnosed BC treated with neoadjuvant chemotherapy.
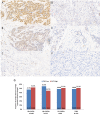
Methods: EVI1 expression was determined by immunohistochemistry using H-score as a cumulative measurement of protein expression in pretherapeutic biopsies of BC patients treated with anthracycline/taxane based neoadjuvant chemotherapy within the GeparTrio trial. EVI1 was analyzed as a continuous variable and dichotomized into low or high based on median expression. Endpoints were pathological complete response (pCR), disease-free survival (DFS) and overall survival (OS).
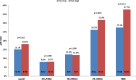
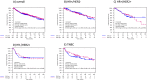
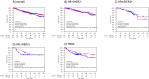
Results: Of the 993 tumors analyzed, 882 had available subtype information: 50.8% were HR + /HER2-, 15% HR + /HER2 + , 9.8% HR-/HER2 + , and 24.5% TNBC. Median EVI1 H-score was 112.16 (range 0.5-291.4). High EVI1 expression was significantly associated with smaller tumor size (p = 0.002) but not with BC subtype. Elevated EVI1 levels were not significantly associated with therapy response and survival in the entire cohort or within BC subtypes. However, TNBC patients with high EVI1 showed a trend towards increased pCR rates compared to low group (37.7% vs 27.5%, p = 0.114; odds ratio 1.60 (95%CI 0.90-2.85, p = 0.110) and numerically better DFS (HR = 0.77 [95%CI 0.48-1.23], log-rank p = 0.271) and OS (HR = 0.76 [95% 0.44-1.31], log-rank p = 0.314) without reaching statistical significance.
Conclusion: EVI1 was not associated with response to neoadjuvant therapy or patient survival in the overall cohort. Further analyses are needed to verify our findings especially in the pathological work-up of early-stage HER2-negative BC patients.
Trial registration: NCT00544765.
Keywords: Breast cancer; EVI1; Neoadjuvant chemotherapy.
© 2022. The Author(s).
Conflict of interest statement
CD reported stock and other ownership interests from Sividon Diagnostics (until 2016); honoraria from Novartis, Roche, MSD Oncology, Daiichi Sankyo, Molecular Health, AstraZeneca, Merck; research funding from Myriad Genetics and Roche; Travel, Accommodations, Expenses frpm Roche; patents, royalties other intellectual property from VMScope digital pathology software; Patent applications WO2015114146A1 and WO2010076322A1- therapy response; Patent application WO2020109570A1—cancer immunotherapy. VM reported personal fees from Amgen, Astra Zeneca, Daiichi-Sankyo, Eisai, Pfizer, MSD, Novartis, Roche, Teva, Seagen and consultancy honoraria from Genomic Health, Hexal, Roche, Pierre Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi-Sankyo, Eisai, Lilly, Tesaro, Nektar, personal fees from Genomic Health, Hexal, Roche, Pierre Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi-Sankyo, Eisai, Lilly, Tesaro and Nektar, other from Novartis, Roche, Seattle Genetics, Genentech, outside the submitted work. BA reported personal fees and non-financial support from Roche, personal fees from Amgen, personal fees from AstraZeneca, personal fees and non-financial support from Tesaro/GSK, personal fees from Clovis, personal fees from Celgene, non-financial support from PharmaMar, personal fees and non-financial support from MSD, personal fees from Novartis, outside the submitted work. AS reported advisory board/speaker's bureau from Astra Zeneca, AGCT, Bayer, BMS, Eli Lilly, Illumina, Janssen, MSD, Novartis, Pfizer, Roche, Seattle Genetics, Takeda, Thermo Fisher; research grants from Bayer, BMS, Chugai, outside the submitted work. SL reported research grants paid to the institution from Abbvie, Amgen, Roche, Celgene, Novartis, Pfizer, other from SeaGen, grants from Immunomedics, other from Prime/Medscape, other from Eirgenix, grants, personal fees and other from DSI, other from BMS, other from Merck, other from Puma, personal fees from Chugai, outside the submitted work; In addition, Dr. Loibl has a patent EP14153692.0 pending. PAF reported personal fees from Novartis, grants from Biontech, personal fees from Pfizer, personal fees from Daiichi-Sankyo, personal fees from Astra Zeneca, personal fees from Eisai, personal fees from Merck Sharp & Dohme, grants from Cepheid, personal fees from Lilly, personal fees from Pierre Fabre, personal fees from Seattle Genetics, personal fees from Roche, personal fees from Hexal, during the conduct of the study. MvM reported personal fees from Amgen, AstraZeneca, Genomic Health, Mylan, Novartis, Pfizer, Pierre Fabre, Roche. FM reported grants from AstraZeneca, personal fees from AstraZeneca, MSD, Clovis, GSK/Tesaro, Pfizer, Novartis, Lilly, Roche, Celgene, Seagen, Myriad, PharmaMar, Eisai, Janssen-Cilag. JUB reported grants from Sysmex, Somatex; personal fees from Amgen, Astra-Zeneca, Lilly, MSD, Novartis, Pfizer, Roche, SonoScape, Sysmex. Other authors have no competing interest.
Figures
References
-
- Gröschel S, Schlenk RF, Engelmann J, Rockova V, Teleanu V, Kühn MW, et al. Deregulated expression of EVI1 defines a poor prognostic subset of MLL-rearranged acute myeloid leukemias: a study of the German-Austrian acute myeloid leukemia study group and the Dutch-Belgian-Swiss HOVON/SAKK cooperative group. J Clin Oncol. 2013;31:95–103. doi: 10.1200/JCO.2011.41.5505. - DOI - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

