Proteomic alterations associated with residual disease in neoadjuvant chemotherapy treated ovarian cancer tissues
- PMID: 36195845
- PMCID: PMC9531351
- DOI: 10.1186/s12014-022-09372-y
Proteomic alterations associated with residual disease in neoadjuvant chemotherapy treated ovarian cancer tissues
Abstract
Background: Optimal cytoreduction to no residual disease (R0) correlates with improved disease outcome for high-grade serous ovarian cancer (HGSOC) patients. Treatment of HGSOC patients with neoadjuvant chemotherapy, however, may select for tumor cells harboring alterations in hallmark cancer pathways including metastatic potential. This study assessed this hypothesis by performing proteomic analysis of matched, chemotherapy naïve and neoadjuvant chemotherapy (NACT)-treated HGSOC tumors obtained from patients who had suboptimal (R1, n = 6) versus optimal (R0, n = 14) debulking at interval debulking surgery (IDS).
Methods: Tumor epithelium was harvested by laser microdissection from formalin-fixed, paraffin-embedded tissues from matched, pre- and post-NACT treated tumors for twenty HGSOC patients and analyzed by quantitative mass spectrometry-based proteomics.
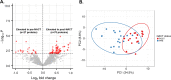
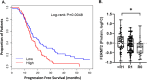
Results: Differential analysis of patient matched pre- and post-NACT treated tumors revealed proteins associated with cell survival and metabolic signaling to be significantly altered in post-NACT treated tumor cells. Comparison of pre-NACT treated tumors from suboptimal (R1) versus optimally (R0) debulked patients identified proteins associated with tumor cell viability and invasion signaling enriched in R1 patients. We identified five proteins altered between R1 and R0 patients in pre- NACT treated tumors that significantly correlated with PFS in an independent cohort of HGSOC patients, including Fermitin family homolog 2 (FERMT2), a protein elevated in R1 that correlated with disease progression in HGSOC patients (multivariate Cox HR = 1.65, Wald p = 0.022) and increased metastatic potential in solid-tumor malignancies.
Conclusions: This study identified distinct proteome profiles in patient matched pre- and post-NACT HGSOC tumors that correlate with NACT resistance and that may predict residual disease status at IDS that collectively warrant further pre-clinical investigation.
Keywords: Neoadjuvant chemotherapy; Ovarian cancer; Personalized medicine, Biomarkers; Proteomics; Residual disease.
© 2022. The Author(s).
Conflict of interest statement
TPC is a ThermoFisher Scientific, Inc SAB member and receives research funding from AbbVie.
Figures

Similar articles
-
Primary debulking surgery versus primary neoadjuvant chemotherapy for high grade advanced stage ovarian cancer: comparison of survivals.Radiol Oncol. 2018 Sep 11;52(3):307-319. doi: 10.2478/raon-2018-0030. Radiol Oncol. 2018. PMID: 30210049 Free PMC article.
-
Optimizing the treatment of ovarian cancer: Neoadjuvant chemotherapy and interval debulking versus primary debulking surgery for epithelial ovarian cancers likely to have suboptimal resection.Gynecol Oncol. 2017 Feb;144(2):266-273. doi: 10.1016/j.ygyno.2016.11.021. Epub 2016 Dec 1. Gynecol Oncol. 2017. PMID: 27916269
-
Neoadjuvant chemotherapy induces phenotypic mast cell changes in high grade serous ovarian cancer.J Ovarian Res. 2024 Sep 28;17(1):192. doi: 10.1186/s13048-024-01516-y. J Ovarian Res. 2024. PMID: 39342316 Free PMC article.
-
[Advanced ovarian cancer: primary debulking (PDS) or neoadjuvant chemotherapy (NACT) - still a debate].Harefuah. 2014 Sep;153(9):527-31, 558. Harefuah. 2014. PMID: 25417489 Review. Hebrew.
-
Systematic Review of the Survival Outcomes of Neoadjuvant Chemotherapy in Women with Malignant Ovarian Germ Cell Tumors.Cancers (Basel). 2023 Sep 8;15(18):4470. doi: 10.3390/cancers15184470. Cancers (Basel). 2023. PMID: 37760440 Free PMC article. Review.
Cited by
-
Challenges and Opportunities for Data Science in Women's Health.Annu Rev Biomed Data Sci. 2023 Aug 10;6:23-45. doi: 10.1146/annurev-biodatasci-020722-105958. Epub 2023 Apr 11. Annu Rev Biomed Data Sci. 2023. PMID: 37040736 Free PMC article. Review.
-
Proteomic alterations in ovarian cancer-Predicting residual disease status using artificial intelligence and SHAP-based biomarker interpretation.Front Med (Lausanne). 2025 Jul 23;12:1562558. doi: 10.3389/fmed.2025.1562558. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40771481 Free PMC article.
-
Proteomic analysis reveals potential biomarker candidates in serous ovarian tumors - a preliminary study.Contemp Oncol (Pozn). 2025;29(1):77-92. doi: 10.5114/wo.2025.149180. Epub 2025 Apr 4. Contemp Oncol (Pozn). 2025. PMID: 40330446 Free PMC article.
References
-
- du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO) Cancer. 2009;115(6):1234–1244. doi: 10.1002/cncr.24149. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
