Metabolic control analysis enables rational improvement of E. coli L-tryptophan producers but methylglyoxal formation limits glycerol-based production
- PMID: 36195869
- PMCID: PMC9531422
- DOI: 10.1186/s12934-022-01930-1
Metabolic control analysis enables rational improvement of E. coli L-tryptophan producers but methylglyoxal formation limits glycerol-based production
Abstract
Background: Although efficient L-tryptophan production using engineered Escherichia coli is established from glucose, the use of alternative carbon sources is still very limited. Through the application of glycerol as an alternate, a more sustainable substrate (by-product of biodiesel preparation), the well-studied intracellular glycolytic pathways are rerouted, resulting in the activity of different intracellular control sites and regulations, which are not fully understood in detail. Metabolic analysis was applied to well-known engineered E. coli cells with 10 genetic modifications. Cells were withdrawn from a fed-batch production process with glycerol as a carbon source, followed by metabolic control analysis (MCA). This resulted in the identification of several additional enzymes controlling the carbon flux to L-tryptophan.
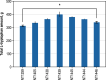
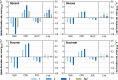
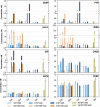
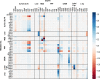
Results: These controlling enzyme activities were addressed stepwise by the targeted overexpression of 4 additional enzymes (trpC, trpB, serB, aroB). Their efficacy regarding L-tryptophan productivity was evaluated under consistent fed-batch cultivation conditions. Although process comparability was impeded by process variances related to a temporal, unpredictable break-off in L-tryptophan production, process improvements of up to 28% with respect to the L-tryptophan produced were observed using the new producer strains. The intracellular effects of these targeted genetic modifications were revealed by metabolic analysis in combination with MCA and expression analysis. Furthermore, it was discovered that the E. coli cells produced the highly toxic metabolite methylglyoxal (MGO) during the fed-batch process. A closer look at the MGO production and detoxification on the metabolome, fluxome, and transcriptome level of the engineered E. coli indicated that the highly toxic metabolite plays a critical role in the production of aromatic amino acids with glycerol as a carbon source.
Conclusions: A detailed process analysis of a new L-tryptophan producer strain revealed that several of the 4 targeted genetic modifications of the E. coli L-tryptophan producer strain proved to be effective, and, for others, new engineering approaches could be derived from the results. As a starting point for further strain and process optimization, the up-regulation of MGO detoxifying enzymes and a lowering of the feeding rate during the last third of the cultivation seems reasonable.
Keywords: Escherichia coli; Glycerol; L-tryptophan; Metabolic control analysis; Methylglyoxal; Thermodynamics-based flux analysis.
© 2022. The Author(s).
Conflict of interest statement
Not applicable.
Figures




References
-
- Weiner M, Tröndle J, Albermann C, Sprenger GA, Weuster-Botz D. Perturbation experiments: approaches for metabolic pathway analysis. Adv Biochem Eng Biotechnol. 2015;152:91–136. - PubMed
-
- Weiner M, Tröndle J, Albermann C, Sprenger GA, Weuster-Botz D. Metabolic control analysis of l-phenylalanine production from glycerol with engineered E. coli using data from short-term steady-state perturbation experiments. Biochem Eng J. 2017;126:86–100. doi: 10.1016/j.bej.2017.06.016. - DOI
-
- Link H, Anselment B, Weuster-Botz D. Rapid media transition: an experimental approach for steady state analysis of metabolic pathways. Biotechnol Prog. 2010;26(1):1–10. - PubMed
-
- Oldiges M, Takors R. Applying metabolic profiling techniques for stimulus-response experiments: chances and pitfalls. Berlin: Springer; 2005. pp. 173–96. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

