Modelling the effects of antimicrobial metaphylaxis and pen size on bovine respiratory disease in high and low risk fattening cattle
- PMID: 36195961
- PMCID: PMC9531528
- DOI: 10.1186/s13567-022-01094-1
Modelling the effects of antimicrobial metaphylaxis and pen size on bovine respiratory disease in high and low risk fattening cattle
Abstract
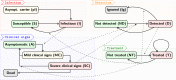
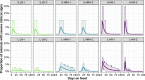
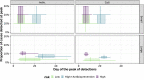
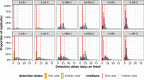
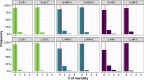
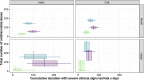
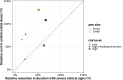
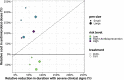
Bovine respiratory disease (BRD) dramatically affects young calves, especially in fattening facilities, and is difficult to understand, anticipate and control due to the multiplicity of factors involved in the onset and impact of this disease. In this study we aimed to compare the impact of farming practices on BRD severity and on antimicrobial usage. We designed a stochastic individual-based mechanistic BRD model which incorporates not only the infectious process, but also clinical signs, detection methods and treatment protocols. We investigated twelve contrasted scenarios which reflect farming practices in various fattening systems, based on pen sizes, risk level, and individual treatment vs. collective treatment (metaphylaxis) before or during fattening. We calibrated model parameters from existing observation data or literature and compared scenario outputs regarding disease dynamics, severity and mortality. The comparison of the trade-off between cumulative BRD duration and number of antimicrobial doses highlighted the added value of risk reduction at pen formation even in small pens, and acknowledges the interest of collective treatments for high-risk pens, with a better efficacy of treatments triggered during fattening based on the number of detected cases.
Keywords: Epidemiological modelling; antimicrobial usage; bovine respiratory disease; disease control; farming practices; stochastic models.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Lechtenberg KF (2019) Bovine respiratory disease modeling. In: Proceedings of the fifty-second annual conference. American Association of Bovine Practitioners. American Association of Bovine Practitioners Proceedings of the Annual Conference, St Louis, Missouri, pp 89–92
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

