Bifidobacterium spp. and their metabolite lactate protect against acute pancreatitis via inhibition of pancreatic and systemic inflammatory responses
- PMID: 36195972
- PMCID: PMC9542615
- DOI: 10.1080/19490976.2022.2127456
Bifidobacterium spp. and their metabolite lactate protect against acute pancreatitis via inhibition of pancreatic and systemic inflammatory responses
Abstract
Severe acute pancreatitis (SAP) is a critical illness characterized by a severe systemic inflammatory response resulting in persistent multiple organ failure and sepsis. The intestinal microbiome is increasingly appreciated to play a crucial role in modulation of AP disease outcome, but limited information is available about the identity and mechanism of action for specific commensal bacteria involved in AP-associated inflammation. Here we show that Bifidobacteria, particularly B. animalis, can protect against AP by regulating pancreatic and systemic inflammation in germ-free (GF) and oral antibiotic-treated (Abx) mouse models. Colonization by B. animalis and administration of its metabolite lactate protected Abx and GF mice from AP by reducing serum amylase concentration, ameliorating pancreatic lesions and improving survival rate after retrograde injection of sodium taurocholate. B. animalis relieved macrophage-associated local and systemic inflammation of AP in a TLR4/MyD88- and NLRP3/Caspase1-dependent manner through its metabolite lactate. Supporting our findings from the mouse study, clinical AP patients exhibited a decreased fecal abundance of Bifidobacteria that was inversely correlated with the severity of systemic inflammatory responses. These results may shed light on the heterogeneity of clinical outcomes and drive the development of more efficacious therapeutic interventions for AP, and potentially for other inflammatory disorders.
Keywords: Bifidobacterium; immunomodulation; lactate; macrophages; microbial metabolite; pancreatic and systemic inflammation.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
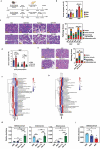

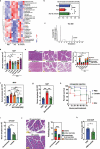

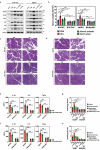
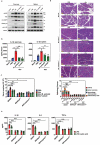

References
-
- Sendler M, Maertin S, John D, Persike M, Weiss FU, Krüger B, Wartmann T, Wagh P, Halangk W, Schaschke N, et al. Cathepsin B activity initiates apoptosis via digestive protease activation in pancreatic acinar cells and experimental pancreatitis. J Biol Chem. 2016;291(28):14717–23. doi:10.1074/jbc.M116.718999. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
