Characterization of sabatolimab, a novel immunotherapy with immuno-myeloid activity directed against TIM-3 receptor
- PMID: 36196369
- PMCID: PMC9525012
- DOI: 10.1093/immadv/ltac019
Characterization of sabatolimab, a novel immunotherapy with immuno-myeloid activity directed against TIM-3 receptor
Erratum in
-
Correction to: Characterization of sabatolimab, a novel immunotherapy with immuno-myeloid activity directed against TIM-3 receptor.Immunother Adv. 2023 Feb 18;3(1):ltad002. doi: 10.1093/immadv/ltad002. eCollection 2023. Immunother Adv. 2023. PMID: 36819977 Free PMC article.
Abstract
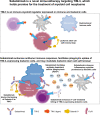
Objectives: Sabatolimab is a humanized monoclonal antibody (hIgG4, S228P) directed against human T-cell immunoglobulin domain and mucin domain-3 (TIM-3). Herein, we describe the development and characterization of sabatolimab.
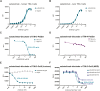
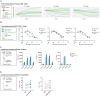
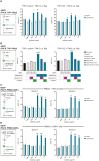
Methods: Sabatolimab was tested for binding to its target TIM-3 and blocking properties. The functional effects of sabatolimab were tested in T-cell killing and myeloid cell cytokine assays. Antibody-mediated cell phagocytosis (ADCP) by sabatolimab was also assessed.
Results: Sabatolimab was shown to (i) enhance T-cell killing and inflammatory cytokine production by dendritic cells (DCs); (ii) facilitate the phagocytic uptake of TIM-3-expressing target cells; and (iii) block the interaction between TIM-3 and its ligands PtdSer/galectin-9.
Conclusion: Taken together, our results support both direct anti-leukemic effects and immune-mediated modulation by sabatolimab, reinforcing the notion that sabatolimab represents a novel immunotherapy with immuno-myeloid activity, holding promise for the treatment of myeloid cell neoplasms.
© The Author(s) 2022. Published by Oxford University Press on behalf of the British Society for Immunology.
Figures

References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
